
Electrochemical Consumables
Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
Item Number : ELCG
Price varies based on specs and customizations
- Airway type
- snake-shaped airway / back-shaped airway / special-shaped custom
- Cell material
- optional PTFE / PEEK / PP / plexiglass / nylon
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
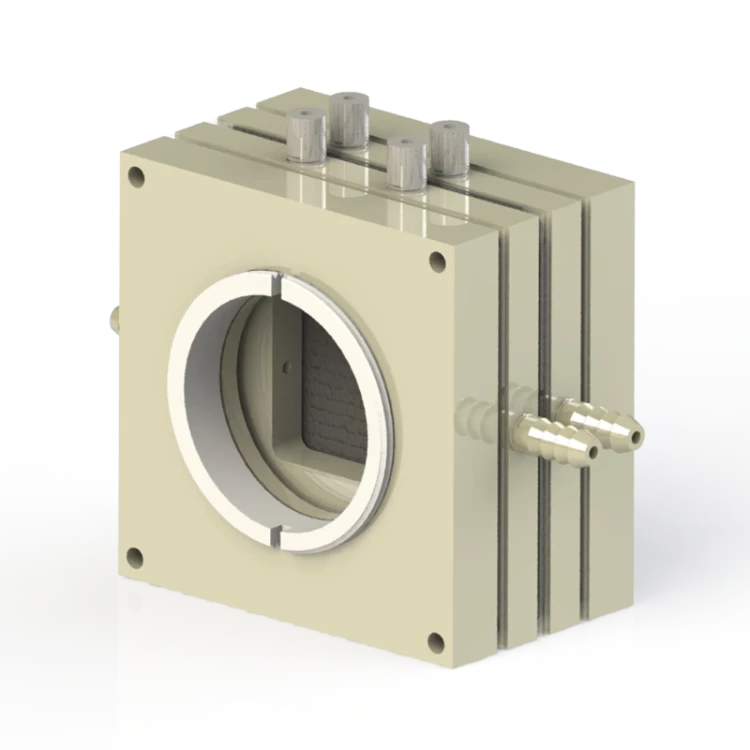
The gas diffusion electrolysis cell is a specialized electrolytic cell that requires confirmation of the customized drawing with us before placing an order. The delivery time for this product is typically between 3 to 6 weeks. It boasts exceptional corrosion resistance and comes in a variety of complete specifications that can also be customized to suit your specific needs.
Customization options
Our gas diffusion electrolysis cells are made of peek, ptfe, pp, plexiglass, nylon, stainless steel, or other materials selected according to electrolyte properties.
We offer customizable functions such as H-type, multi-connected H-type, monomer, one/two-way optical gas flow cell, liquid flow cell, and solid-liquid reaction cell.
Our self-made electrodes can be made of platinum wire/mesh/sheet, gold sheet, or graphite.
We use silica gel, fluorine rubber, or PTFE gasket for sealing.
Technical specifications
| Airway type | snake-shaped airway / back-shaped airway / special-shaped custom |
| Cell material | optional PTFE / PEEK / PP / plexiglass / nylon |
Detail & Parts
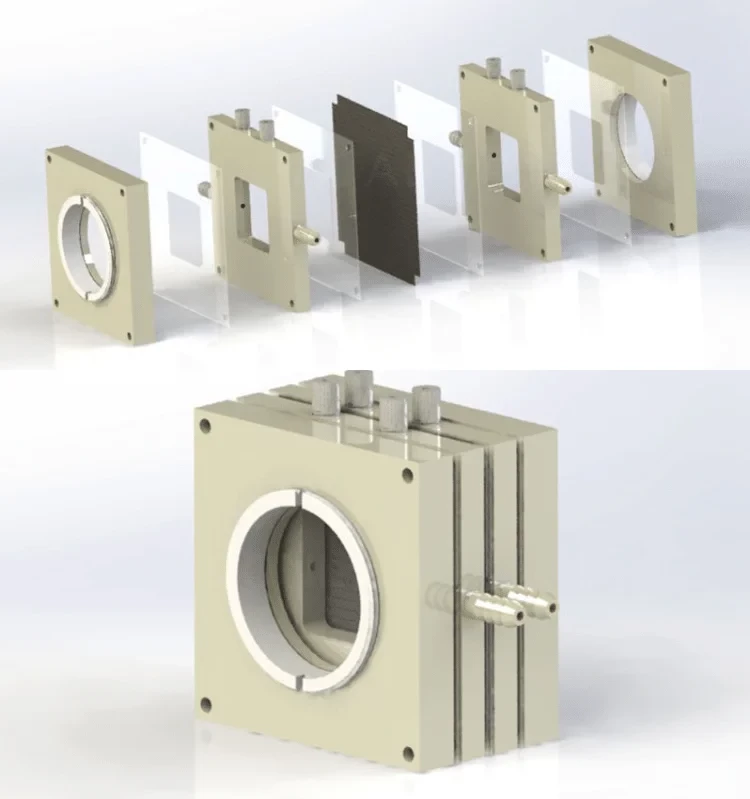







Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Are Electrolytic Cells Used For?
What Are The Materials Used In Electrochemical Cell?
What Is The Difference Between Galvanic Cell And Electrolytic Cell?
What Are The Examples Of Electrochemical Material?
What Is An Electrolytic Cell And How Does It Work?
What Are The Two Points Of Difference Between Electrochemical And Electrolytic Cells?
What Is The Example Of Electrolytic Cell?
Are Electrolytic Cells Spontaneous?
4.8 / 5
The customizability of this cell is amazing. We were able to get exactly what we needed for our research.
4.9 / 5
The quality of this cell is top-notch. It's well-made and durable, and it's holding up well to our rigorous testing.
4.7 / 5
The delivery time was very fast, and the cell arrived in perfect condition. We're very happy with the service we received.
5.0 / 5
This cell is a great value for the money. It's affordable, but it doesn't sacrifice quality.
4.8 / 5
We've been using this cell for a few months now, and it's been working flawlessly. We're very happy with it.
4.9 / 5
The technological advancements in this cell are impressive. It's a cutting-edge piece of equipment that's helping us to push the boundaries of our research.
4.7 / 5
The customer service we received from KINTEK SOLUTION was excellent. They were very helpful and responsive to our questions.
5.0 / 5
This cell is a game-changer for our research. It's allowing us to do things that we couldn't do before.
4.8 / 5
The delivery time was very fast, and the cell arrived in perfect condition. We're very happy with the service we received.
4.9 / 5
The quality of this cell is top-notch. It's well-made and durable, and it's holding up well to our rigorous testing.
4.7 / 5
The customizability of this cell is amazing. We were able to get exactly what we needed for our research.
5.0 / 5
This cell is a great value for the money. It's affordable, but it doesn't sacrifice quality.
4.8 / 5
We've been using this cell for a few months now, and it's been working flawlessly. We're very happy with it.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.

Electrolytic Electrochemical Cell for Coating Evaluation
Looking for corrosion-resistant coating evaluation electrolytic cells for electrochemical experiments? Our cells boast complete specifications, good sealing, high-quality materials, safety, and durability. Plus, they're easily customizable to meet your needs.

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.

Optical Water Bath Electrolytic Electrochemical Cell
Upgrade your electrolytic experiments with our Optical Water Bath. With controllable temperature and excellent corrosion resistance, it's customizable for your specific needs. Discover our complete specifications today.

Flat Corrosion Electrolytic Electrochemical Cell
Discover our flat corrosion electrolytic cell for electrochemical experiments. With exceptional corrosion resistance and complete specifications, our cell guarantees optimal performance. Our high-quality materials and good sealing ensure a safe and durable product, and customization options are available.

Super Sealed Electrolytic Electrochemical Cell
Super-sealed electrolytic cell offers enhanced sealing capabilities, making it ideal for experiments that require high airtightness.

Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Discover our high-quality Multifunctional Electrolytic Cell Water Baths. Choose from single or double-layer options with superior corrosion resistance. Available in 30ml to 1000ml sizes.

Lab Electrochemical Workstation Potentiostat for Laboratory Use
Electrochemical workstations, also known as laboratory electrochemical analyzers, are sophisticated instruments designed for precise monitoring and control in various scientific and industrial processes.

RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Elevate your electrochemical research with our Rotating Disk and Ring Electrodes. Corrosion resistant and customizable to your specific needs, with complete specifications.
Related Articles

Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
Electrolytic cells are devices that utilize an electric current to induce a non-spontaneous chemical reaction.

Applications of H-Type Electrolytic Cell in Metal Extraction
H-type electrolytic cells uses an electrolyte solution to dissolve the metal ions and an electric current to separate the metal ions from the solution.

The Silent Dialogue: Mastering Control in Electrolytic Cells
Electrolysis is a non-spontaneous act requiring precise control. Learn to interpret the interplay of voltage, current, and physical phenomena for safer lab results.

The Vessel of Truth: Why the Container Matters More Than the Chemistry
The success of an electrolytic experiment often hangs on the material of the cell body. Discover the trade-offs between Borosilicate, Quartz, and PTFE.

Overcoming Challenges with H-Type Electrolytic Cell Operation
Understanding the components and operation of the H-type electrolytic cell is crucial in producing high-quality chemicals and overcoming the challenges that come with its operation.

Electrochemistry The Science Behind Electrochemical Cells
Electrochemistry is important because it helps us understand the behavior of materials and substances in different environments.

Electrochemical Cells: Generating Electricity and Driving Reactions
Electrochemical cells, like batteries, play a vital role in energy storage by converting chemical energy to electrical energy and vice versa. Explore the workings, types, and significance of these cells.

Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
Explore the detailed workings of flat corrosion electrolytic cells, their role in industrial processes, and effective strategies to mitigate corrosion. Learn about electrolytic cells, their components, and applications in electroplating and metal purification.

The Quiet Discipline: Mastering the Post-Use Protocol for Five-Port Electrolytic Cells
Learn the methodical post-use care for five-port water bath electrolytic cells. Prevent corrosion, ensure safety, and protect your experimental data.

Benefits of Electrochemical Cells for Energy Storage
Electrochemical cells are devices that convert chemical energy into electrical energy through the use of oxidation-reduction reactions. They are widely used in various applications such as energy storage, fuel cells, and batteries.

The Geometry of Control: Why 1 cm² Defines Electrochemical Success
Discover why the standard 1 cm² reaction area and precision O-ring sealing create the necessary baseline for repeatable, accurate electrochemical data.

The Silent Variable: Engineering Reliability in Electrolytic Cells
Data accuracy depends on equipment integrity. Learn the engineering protocols for maintaining electrolytic cells to prevent systemic error.