
Electrochemical Consumables
Flat Corrosion Electrolytic Electrochemical Cell
Item Number : ELEFC
Price varies based on specs and customizations
$799.00 / set
- Specifications
- 350ml, can be customized
- Applicable temperature range
- 0 ~ 100℃
- Material
- boron glass + PTFE
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
We offer a flat corrosion electrochemical cell that is ideal for conducting electrochemical experiments. Our cell boasts exceptional corrosion resistance and comes with complete specifications, ensuring optimal performance. Additionally, we prioritize good sealing and high-quality material selection, resulting in a safe and durable product. Customization options are also available.
Technical specifications
Flat corrosion electrolytic cell
| Specifications | 350ml, can be customized |
| Applicable temperature range | 0 ~ 70℃ |
| Sealing form | TSilicone rubber gasket |
| Material | boron glass + PTFE |
| Hole | three grinding mouths + two inner circulation pagoda mouths |
Plate corrosion electrolytic cell - double layer with water bath
| Specifications | 350ml, can be customized |
| Applicable temperature range | 0 ~ 100℃ |
| Material | boron glass + PTFE |
| Hole | Three grinding mouth two circulation + water bath |
Detail & Parts



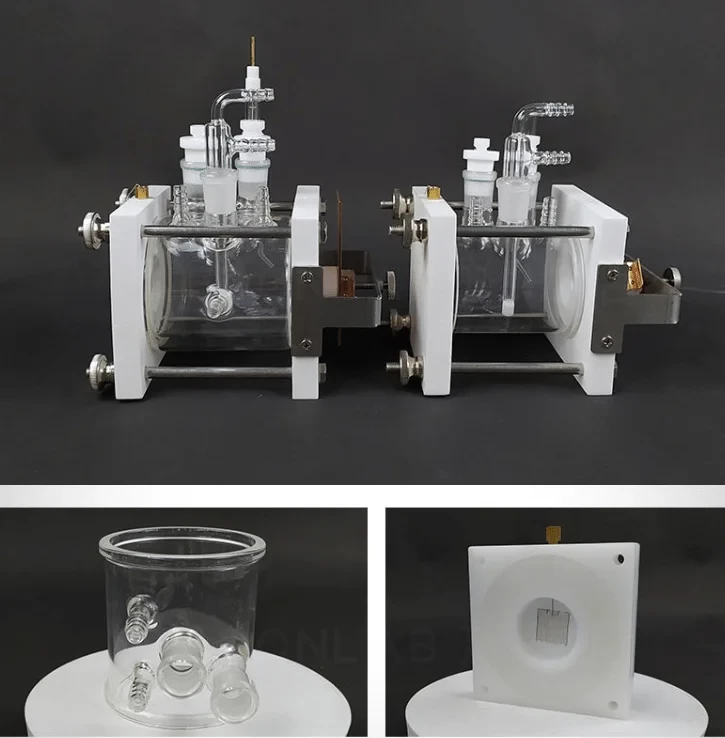

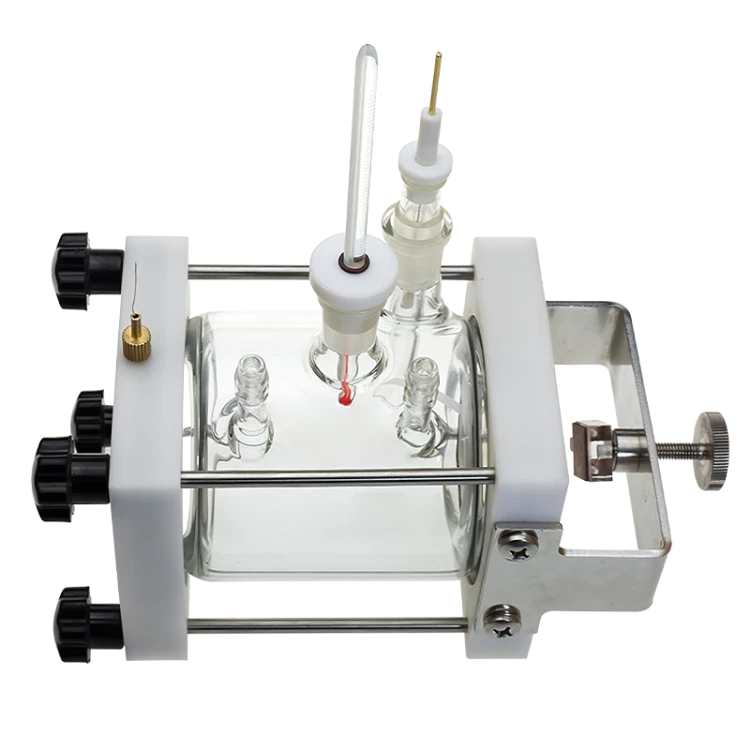

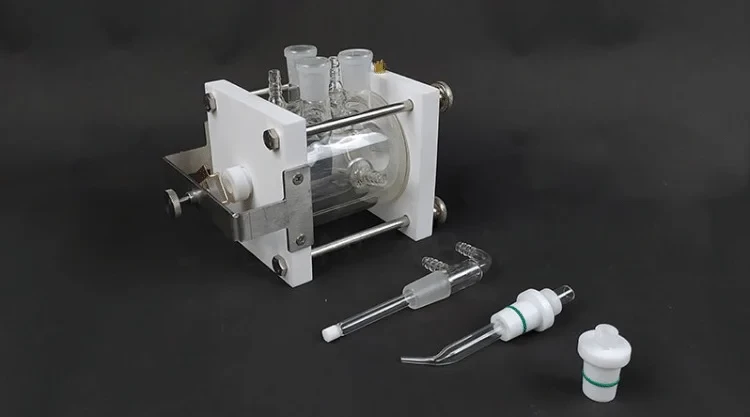



Operating steps

1. Prepare the cell body and its accessories, as well as the sample that will be tested.

2. During installation, install the aeration pipe, salt bridge, and thermometer into the pool body.

3. To make room for the sample piece, unscrew the working electrode fixing cap backwards and place the sealing gasket on it.

4. Allow the sample to adhere to the sealing gasket, then tighten the fixing cap to ensure that the test can be performed without any leaks.

5. The installation process is now complete.
Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Are Electrolytic Cells Used For?
What Are The Materials Used In Electrochemical Cell?
What Is The Difference Between Galvanic Cell And Electrolytic Cell?
What Are The Examples Of Electrochemical Material?
What Is An Electrolytic Cell And How Does It Work?
What Are The Two Points Of Difference Between Electrochemical And Electrolytic Cells?
What Is The Example Of Electrolytic Cell?
Are Electrolytic Cells Spontaneous?
4.9 / 5
I appreciate the quick delivery of the flat corrosion electrolytic cell! It arrived just in time for my experiment.
4.7 / 5
The flat corrosion electrolytic cell is a great value for the price. It's well-made and has all the features I need.
4.8 / 5
I'm impressed with the quality of the flat corrosion electrolytic cell. It's durable and has held up well in my lab.
4.7 / 5
The flat corrosion electrolytic cell is a valuable addition to my lab equipment. It's easy to use and gives me accurate results.
4.9 / 5
I'm very happy with the flat corrosion electrolytic cell. It's a great tool for my research.
4.8 / 5
The flat corrosion electrolytic cell is a must-have for any lab. It's versatile and can be used for a variety of experiments.
4.7 / 5
I'm impressed with the technological advancement of the flat corrosion electrolytic cell. It's a powerful tool that has helped me make significant progress in my research.
4.9 / 5
The flat corrosion electrolytic cell is a game-changer for my lab. It's made my experiments more efficient and accurate.
4.8 / 5
I highly recommend the flat corrosion electrolytic cell to other researchers. It's a great investment that will pay for itself in no time.
4.7 / 5
The flat corrosion electrolytic cell is a must-have for any lab that conducts electrochemical experiments.
4.9 / 5
I'm very satisfied with the flat corrosion electrolytic cell. It's a reliable and accurate piece of equipment.
4.8 / 5
The flat corrosion electrolytic cell is a great addition to my lab. It's easy to use and gives me consistent results.
4.7 / 5
I'm impressed with the quality and performance of the flat corrosion electrolytic cell. It's a valuable tool for my research.
4.9 / 5
The flat corrosion electrolytic cell is a great investment for my lab. It's helped me save time and money.
4.7 / 5
I'm very happy with the flat corrosion electrolytic cell. It's a great tool for my research.
4.8 / 5
The flat corrosion electrolytic cell is a great choice for my lab. It's durable and easy to use.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
Looking for a high-quality gas diffusion electrolysis cell? Our liquid flow reaction cell boasts exceptional corrosion resistance and complete specifications, with customizable options available to suit your needs. Contact us today!

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.

Electrolytic Electrochemical Cell for Coating Evaluation
Looking for corrosion-resistant coating evaluation electrolytic cells for electrochemical experiments? Our cells boast complete specifications, good sealing, high-quality materials, safety, and durability. Plus, they're easily customizable to meet your needs.

Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Looking for a reliable quartz electrochemical cell? Our product boasts excellent corrosion resistance and complete specifications. With high-quality materials and good sealing, it's both safe and durable. Customize to meet your needs.

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.

Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Discover our high-quality Multifunctional Electrolytic Cell Water Baths. Choose from single or double-layer options with superior corrosion resistance. Available in 30ml to 1000ml sizes.

H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Double-layer H-type optical water bath electrolytic cells, with excellent corrosion resistance and a wide range of specifications available. Customization options are also available.

Super Sealed Electrolytic Electrochemical Cell
Super-sealed electrolytic cell offers enhanced sealing capabilities, making it ideal for experiments that require high airtightness.

Optical Water Bath Electrolytic Electrochemical Cell
Upgrade your electrolytic experiments with our Optical Water Bath. With controllable temperature and excellent corrosion resistance, it's customizable for your specific needs. Discover our complete specifications today.

Electrode Polishing Material for Electrochemical Experiments
Looking for a way to polish your electrodes for electrochemical experiments? Our polishing materials are here to help! Follow our easy instructions for best results.
Related Articles

Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
Explore the detailed workings of flat corrosion electrolytic cells, their role in industrial processes, and effective strategies to mitigate corrosion. Learn about electrolytic cells, their components, and applications in electroplating and metal purification.

Advanced Techniques in Coating Evaluation Using Electrolytic Cells
Explore the comprehensive guide on coating evaluation using electrolytic cells, covering electroplating, sol-gel methods, and wet chemical techniques. Enhance your understanding of metal coating properties and applications.

Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
Explore the detailed guide on saturated calomel reference electrodes, including their composition, advantages, disadvantages, and applications. Ideal for researchers and lab technicians.

Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
Electrolytic cells are devices that utilize an electric current to induce a non-spontaneous chemical reaction.

Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits
Discover the versatile applications of multifunctional electrolytic cell water baths in various industries. Learn about their benefits, components, and how they facilitate chemical reactions and temperature control.

Understanding Electrodes and Electrochemical Cells
An electrode is a point where current enters and leaves the electrolyte. It is a conductor used to make a junction with a nonmetallic part of a circuit. Electrodes can be made of materials such as gold, platinum, carbon, graphite, or metal. They serve as the surface for oxidation-reduction reactions in electrochemical cells. There are different types of electrodes, including anode and cathode.

Applications of Electrolytic Cells in Purification and Electroplating
Electrolytic cells are chemical cells that use electricity to generate a non-spontaneous redox reaction. These cells are used in various electrochemical processes such as electrolysis and electroplating.

Understanding Electrodeposition with Electrochemical Electrodes
Electrodeposition is a process of depositing a metal or a non-metallic material onto a surface by applying an electric current.

Applications of H-Type Electrolytic Cell in Metal Extraction
H-type electrolytic cells uses an electrolyte solution to dissolve the metal ions and an electric current to separate the metal ions from the solution.

How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
A reference electrode is an electrode with a stable and well-defined potential that is used as a reference point to measure the potential of other electrodes. Reference electrodes are commonly used in electrochemical experiments to determine the potential difference between two electrodes.

Electrochemistry The Science Behind Electrochemical Cells
Electrochemistry is important because it helps us understand the behavior of materials and substances in different environments.

How to Choose the Right Electrochemical Electrode
The choice of electrode material can have a significant impact on the performance of the electrochemical system.