Introduction to Multifunctional Electrolytic Cell Water Baths
Multifunctional electrolytic cell water baths are pivotal tools in both scientific research and industrial applications, offering a versatile platform for a myriad of processes. These advanced systems are designed to facilitate chemical reactions and maintain precise temperature control, making them indispensable in fields ranging from bacteriological examinations to environmental studies. Understanding the basic principles of operation, key components, and the myriad applications of these water baths is essential for researchers and industry professionals alike. This article delves into the intricacies of multifunctional electrolytic cell water baths, exploring their construction, functionality, and the benefits they bring to various sectors.
Key Components of Electrolytic Cell Water Baths
Electrolytic cell water baths are essential tools in various scientific and industrial applications, particularly in processes that require the decomposition of compounds through electrical energy. These systems are composed of several key components: the cathode, anode, electrolyte, and the power source. Each component plays a crucial role in the electrolytic process, facilitating the transfer of electrons and the subsequent chemical reactions.
The Cathode
The cathode is one of the two electrodes in an electrolytic cell and is negatively charged. During the electrolytic process, the cathode attracts positively charged ions (cations) from the electrolyte. These cations gain electrons at the cathode surface in a process called reduction. Reduction is a type of chemical reaction where electrons are added to an atom or ion, thereby decreasing its oxidation state. For example, in the electrolysis of water, hydrogen ions (H+) from the water molecule are reduced to form hydrogen gas (H₂), which is released as a byproduct.
The Anode
Opposite to the cathode, the anode in an electrolytic cell is positively charged. It attracts negatively charged ions (anions) from the electrolyte. At the anode, these anions lose electrons in a process known as oxidation. Oxidation involves the removal of electrons from an atom or ion, thereby increasing its oxidation state. In the electrolysis of water, hydroxide ions (OH⁻) are oxidized at the anode to produce oxygen gas (O₂) and water.
The Electrolyte
The electrolyte is a critical component of an electrolytic cell as it facilitates the conduction of electricity. It is typically a solution containing dissolved ions, which can be either aqueous solutions like water with dissolved salts or molten salts. The ions in the electrolyte move freely and carry electrical charges between the electrodes. In the case of water electrolysis, the electrolyte is often a dilute solution of sulfuric acid or sodium hydroxide, which increases the ionic concentration and enhances the conductivity of the water.
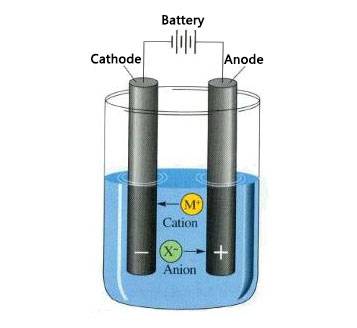
The Power Source
The power source, often a battery or a direct current (DC) power supply, provides the necessary electrical energy to drive the non-spontaneous redox reactions in the electrolytic cell. It applies a voltage across the electrodes, creating an electric field that forces the ions in the electrolyte to move. The movement of these ions towards the respective electrodes initiates the reduction and oxidation reactions, leading to the decomposition of the compound in the electrolyte.
Role of Each Component in the Electrolytic Process
Each component of the electrolytic cell plays a vital role in the overall process:
- Cathode: Acts as the site for reduction reactions, where cations gain electrons.
- Anode: Serves as the site for oxidation reactions, where anions lose electrons.
- Electrolyte: Conducts electricity by allowing the movement of ions between the electrodes.
- Power Source: Supplies the electrical energy required to initiate and sustain the redox reactions.
In summary, understanding the function and interaction of these components is essential for optimizing the performance of electrolytic cell water baths in various applications, from industrial production of chemicals to laboratory research. Each component's role is intricately linked, ensuring efficient and controlled electrolytic processes.
Applications of Electrolytic Cell Water Baths
Electrolytic cell water baths play a crucial role in various scientific and industrial applications, facilitating a range of processes from simple heating to complex chemical reactions. These versatile devices are essential in fields such as bacteriological examinations, food processing, microbiology assays, and environmental studies. Below, we delve into the specific applications and benefits of electrolytic cell water baths across different sectors.
Bacteriological Examinations
In bacteriological examinations, electrolytic cell water baths are used for maintaining precise temperatures required for bacterial growth and analysis. These baths provide a stable environment that mimics natural conditions, ensuring accurate results in tests such as bacterial culturing and antibiotic susceptibility testing. The ability to maintain constant temperatures is crucial as fluctuations can significantly affect bacterial behavior and test outcomes.
Food Processing
In the food industry, electrolytic cell water baths are employed in various stages of food processing and quality control. They are used for tasks such as pasteurization, where maintaining a specific temperature is critical to kill harmful bacteria without compromising the food's quality. Additionally, these baths are used in the preparation of samples for sensory analysis and nutritional testing, ensuring that the samples are consistently heated to the required temperatures.
Microbiology Assays
Microbiology assays often require controlled temperature environments to study microbial growth, enzyme activity, and other biological processes. Electrolytic cell water baths provide the necessary stability and precision, making them indispensable in research laboratories. They are used in assays to determine the effectiveness of disinfectants, study the growth of pathogenic microorganisms, and investigate the metabolic activities of beneficial microbes.
Environmental Studies
Environmental studies utilize electrolytic cell water baths for a variety of applications, including the analysis of water and soil samples. These baths help in maintaining optimal temperatures for chemical reactions, microbial growth, and other environmental processes that are temperature-sensitive. They are particularly useful in studies related to pollution monitoring, biodegradation processes, and climate change effects on ecosystems.
Industrial Applications
Beyond the laboratory, electrolytic cell water baths have significant industrial applications. They are used in the production of certain chemicals, pharmaceuticals, and materials where precise temperature control is essential. For instance, in the synthesis of polymers and other organic compounds, these baths ensure that reactions proceed at the correct rates and under controlled conditions, leading to high-quality end products.
Advantages of Electrolytic Cell Water Baths
The primary advantage of electrolytic cell water baths lies in their ability to provide uniform and stable temperatures over extended periods. This is achieved through advanced digital control systems that offer greater temperature uniformity, stability, and control. These features make them ideal for applications requiring consistent heating or cooling, such as in research, quality control, and industrial processes.
In conclusion, electrolytic cell water baths are versatile tools with applications spanning multiple scientific and industrial fields. Their ability to maintain precise temperatures makes them invaluable in bacteriological examinations, food processing, microbiology assays, and environmental studies. As technology advances, these baths continue to evolve, offering enhanced capabilities and greater efficiency, thereby expanding their utility in various sectors.
Temperature Control and Stability in Electrolytic Cell Water Baths
Temperature control and stability in electrolytic cell water baths are critical for maintaining precise experimental conditions. Digital control systems play a pivotal role in enhancing temperature uniformity and stability, ensuring that experiments yield accurate and reliable results. This section delves into the intricacies of digital temperature control systems and their impact on experimental outcomes.
Importance of Temperature Uniformity and Stability
Temperature uniformity refers to the consistency of temperature across the entire bath, while stability denotes the ability to maintain a constant temperature over time. Both factors are crucial for experiments that require precise temperature conditions. For instance, in bacteriological examinations, even minor temperature fluctuations can affect the growth rate of microorganisms, leading to inaccurate results. Similarly, in food processing and quality control procedures, maintaining a stable temperature is essential for ensuring consistent product quality.
Digital Control Systems: Enhancing Uniformity and Stability
Digital control systems offer several advantages over traditional analog systems. These include:
-
Precision Control: Digital systems provide more precise control over temperature settings. They allow for fine-tuning of temperature set points, ensuring that the bath remains at the desired temperature within a narrow range. This precision is particularly important in applications where small temperature differences can have significant impacts, such as in microbiological assays.
-
Real-Time Monitoring: Digital systems often come with real-time monitoring capabilities, allowing users to track temperature fluctuations instantly. This feature enables prompt adjustments to be made if deviations occur, thereby maintaining stability.
-
Advanced Algorithms: Many digital control systems utilize advanced algorithms to optimize temperature distribution within the bath. These algorithms can adjust heating and cooling rates dynamically, ensuring uniform temperature across the bath. For example, some systems can scale each output at specified temperatures, improving uniformity at required set points.
-
Consistency: Digital systems are less prone to human error compared to manual controls. They can maintain consistent temperature settings over extended periods, reducing the likelihood of experimental variability.
Practical Applications of Digital Control Systems
Digital control systems are employed across various applications, including:
-
Bacteriological Examinations: In microbiology labs, digital water baths are used for culturing bacteria. The precise temperature control ensures optimal growth conditions, facilitating accurate identification and analysis of bacterial strains.
-
Food Processing and Quality Control: In the food industry, digital water baths are used for processes such as pasteurization and sterilization. The stability and uniformity of temperature are crucial for maintaining food safety and quality standards.
-
Microbiological Assays: Digital water baths are essential for conducting various microbiological assays, such as enzyme-linked immunosorbent assays (ELISAs). The precise temperature control ensures that reactions proceed at optimal rates, leading to accurate assay results.

Considerations for Choosing a Digital Water Bath
When selecting a digital water bath, several factors should be considered:
-
Temperature Range: Ensure that the bath can operate within the required temperature range for your experiments. Some digital baths offer a wide range of temperatures, from sub-zero to high temperatures, catering to diverse applications.
-
Accuracy and Stability: Look for baths that offer high accuracy and stability. A temperature stability within ±0.2 degrees Celsius is generally acceptable for most applications. However, for more critical experiments, higher precision may be necessary.
-
Uniformity: Check the bath's ability to maintain temperature uniformity. Stirred units are typically better at achieving high uniformity compared to unstirred units, which can be affected by convection currents.
-
Ease of Use: Consider the user interface of the digital control system. A user-friendly interface with clear displays and intuitive controls can simplify the operation and monitoring of the bath.
-
Durability and Maintenance: Choose a bath that is built to last and requires minimal maintenance. High-quality materials and robust construction can ensure long-term reliability and reduce downtime.
Conclusion
Digital control systems significantly enhance temperature uniformity and stability in electrolytic cell water baths, making them indispensable for modern laboratories. By providing precise control, real-time monitoring, and advanced algorithms, digital systems ensure that experiments are conducted under optimal conditions, leading to accurate and reliable results. When selecting a digital water bath, it is crucial to consider factors such as temperature range, accuracy, uniformity, ease of use, and durability to meet the specific needs of your experiments.
Safety Features and Insulation in Electrolytic Cell Water Baths
Electrolytic cell water baths are essential tools in various laboratory settings, including industrial, clinical, academic, and government research laboratories. These baths are used for a wide range of applications such as sample thawing, bacteriological examinations, warming reagents, and microbiological assays. Given their widespread use and the critical nature of their applications, ensuring safety and efficiency is paramount. This section delves into the safety features, insulation, and ATEX-compliant heaters that are integral to the design and operation of electrolytic cell water baths.
Insulation and Cool-Touch Exteriors
One of the primary safety features of electrolytic cell water baths is their insulation and cool-touch exteriors. The baths are typically constructed with a rounded, seamless stainless steel reservoir that is resistant to rust, chemical damage, and contamination. The exterior is often epoxy-powder coated, which not only simplifies cleanup but also ensures that the surface remains cool to the touch, even after extended use. This design prevents accidental burns and enhances user safety.
ATEX-Compliant Heaters
The heaters used in electrolytic cell water baths are often ATEX-compliant, which means they meet the stringent safety standards for use in explosive atmospheres. These heaters are designed to prevent the ignition of flammable gases and dust, ensuring a safe operating environment. Depending on the application area’s Hazardous Zone Classification, the heaters can also be weather-proof, providing additional protection against environmental factors.
Secondary Thermostats and Safety Alarms
To further enhance safety, electrolytic cell water baths are equipped with secondary thermostats that automatically disconnect the heater power if the bath temperature exceeds safe limits or if the liquid level drops too low. This feature prevents overheating and potential damage to the equipment. Additionally, alarm indicators are included to alert users of any abnormal conditions, ensuring prompt response and mitigation of risks.
Non-Contact Recessed Heating Elements
The heating elements in these baths are designed to be non-contact and recessed, which helps in curtailing element burnout and eliminating tank hot spots. This design ensures uniform heating and extends the lifespan of the equipment. The absence of direct contact between the heating elements and the bath liquid also reduces the risk of contamination and enhances the overall efficiency of the heating process.
Digital Control Systems
Modern electrolytic cell water baths are equipped with advanced digital control systems that provide greater temperature uniformity, stability, and control. These systems operate from ambient temperature to 99°C (210°F) with PID temperature control stepping at 0.1°C increments. The digital controls ensure precise temperature management, which is crucial for various laboratory applications such as bacteriological examinations, food processing/QC procedures, and microbiology assays.

Overheat Protection and User Safety
Safety is a top priority in the design of electrolytic cell water baths. Built-in overheat protectors automatically shut down power if the controller fails, preventing potential hazards. Users are also advised to exercise caution when operating the baths, especially when using glass reaction equipment. Precautions must be taken to avoid contact with rotating parts and to prevent entanglement of loose clothing, hair, or jewelry. Extra caution is required when operating with air-reactive materials, particularly under vacuum conditions, to avoid violent reactions.
Rotary Flask and Evaporating Flask
The rotary flask, a key component of the electrolytic cell water bath, is typically made of Borosilicate Glass 3.3 raw material. This material is chosen for its durability and resistance to thermal shock. The flask’s construction is critical for smooth rotation along the central axis, ensuring that the entire surface area comes in contact with the heating media in the bath for uniform heating. Advanced manufacturing techniques ensure that the evaporating flask rotates perfectly along the central axis, maximizing efficiency and safety.
In conclusion, electrolytic cell water baths are designed with a multitude of safety features to ensure user safety and equipment longevity. These features include insulation, cool-touch exteriors, ATEX-compliant heaters, secondary thermostats, non-contact recessed heating elements, digital control systems, and overheat protection. By incorporating these advanced safety measures, electrolytic cell water baths provide a reliable and safe environment for a wide range of laboratory applications.
Electrolysis Process in Water Baths
Electrolysis is a fundamental process in chemistry and industry, particularly in water baths, where it involves the use of direct electric current (DC) to drive non-spontaneous redox reactions. This process is crucial in various applications, from the decomposition of water to produce hydrogen and oxygen, to the extraction of metals through the electrolysis of molten salts. Understanding the flow of electrons and the role of the electrolytic cell is essential for harnessing the power of electrolysis.
The Electrolytic Cell: Structure and Function
An electrolytic cell consists of three primary components: an electrolyte and two electrodes, the cathode and the anode. The electrolyte is typically a solution of dissolved ions in water or other solvents, which can also include molten salts like sodium chloride. When an external voltage is applied across the electrodes, the ions in the electrolyte are attracted to the electrode with the opposite charge, facilitating charge-transferring (redox) events.
The cathode, which is negatively charged, attracts positive ions (cations), while the anode, positively charged, attracts negative ions (anions). This movement of ions across the electrolyte is the basis of the electrolytic process. The electrolyte serves as a conductive medium, allowing the flow of ions between the electrodes, which is crucial for the redox reactions to occur.
Mechanism of Electrolysis
The process of electrolysis can be explained through the principle of ionization. According to ionic theory, electrolytes exist as ions in solution, and the function of electricity is to direct these ions towards their respective electrodes. Electrolytes can be electrolyzed only in the dissolved or molten state.
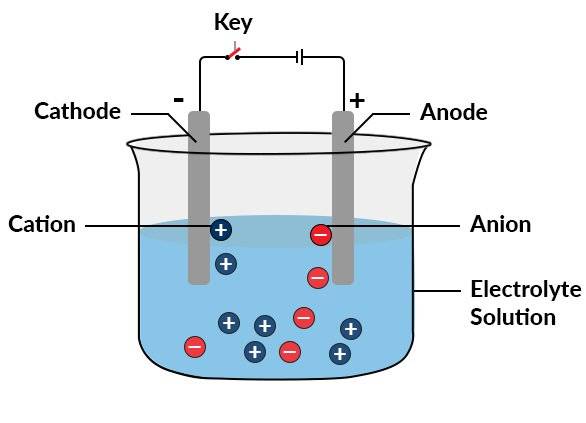
In a typical setup, such as two copper rods dipped in an aqueous solution of copper sulfate, when an electric current (DC voltage) is applied, Cu2+ ions discharge at the cathode (negatively charged electrode), and the following reaction occurs:
At cathode: Cu2+ + 2e- → Cu (reduction)
Simultaneously, at the anode, a corresponding oxidation reaction takes place, completing the redox process.
Applications of Electrolysis in Water Baths
One of the most common applications of electrolysis in water baths is the decomposition of water to produce hydrogen and oxygen gases. This process is achieved by utilizing the flow of electrons to overcome the activation energy barrier of the non-spontaneous redox reaction. The reactions at the electrodes are as follows:
At cathode: 2H2O + 2e- → H2 + 2OH- At anode: 2H2O → O2 + 4H+ + 4e-
This method of water electrolysis is not only a clean source of hydrogen but also a demonstration of how electrolytic cells can facilitate complex chemical transformations.
Conclusion
The electrolysis process in water baths is a powerful tool in both scientific research and industrial applications. By understanding the flow of electrons and the role of the electrolytic cell, we can harness the energy of non-spontaneous redox reactions to produce valuable substances like hydrogen and to extract metals from their ores. The versatility and efficiency of electrolytic cells make them indispensable in modern chemistry and technology.
Future Trends and Innovations in Electrolytic Cell Water Baths
The field of electrolytic cell water baths has seen significant advancements, driven by the need for more precise and efficient temperature control in various research and development applications. As technology continues to evolve, several emerging trends and innovations promise to further enhance the functionality and efficiency of these essential laboratory tools.
Integration of Smart Technologies
One of the most promising trends in electrolytic cell water baths is the integration of smart technologies. Modern water baths are increasingly equipped with advanced sensors and digital interfaces that allow for real-time monitoring and control of temperature, pH, and other critical parameters. These smart features not only improve precision but also enable remote operation and data logging, facilitating better experimental management and reproducibility.
For instance, some advanced water baths now come with built-in Wi-Fi connectivity, allowing researchers to monitor and adjust settings from their smartphones or tablets. This level of connectivity can significantly streamline laboratory workflows, reducing the time and effort required for manual interventions.
Energy Efficiency and Sustainability
Energy efficiency is another critical area of innovation in electrolytic cell water baths. Traditional water baths can be energy-intensive, especially when maintaining high temperatures over extended periods. However, recent advancements have focused on developing more sustainable solutions that reduce energy consumption without compromising performance.
One approach is the use of advanced insulation materials and heat recovery systems. These innovations help minimize heat loss, ensuring that the water bath maintains its set temperature with less energy input. Additionally, some manufacturers are exploring the use of renewable energy sources, such as solar power, to further reduce the environmental impact of these devices.
Enhanced Safety Features
Safety is paramount in any laboratory setting, and electrolytic cell water baths are no exception. Emerging innovations in this field are aimed at enhancing safety features to protect both researchers and equipment. For example, modern water baths often include automatic shut-off mechanisms that trigger in case of overheating or fluid leaks, preventing potential accidents.
Moreover, some water baths now come with built-in safety covers that prevent accidental splashes and reduce evaporation, thereby maintaining a cleaner and safer working environment. These safety enhancements not only protect researchers but also help extend the lifespan of the equipment by reducing exposure to harmful chemicals and contaminants.

Customization and Modularity
As research needs become more specialized, there is a growing demand for customizable and modular electrolytic cell water baths. This trend involves designing water baths that can be easily modified or expanded to accommodate different experimental requirements. For instance, some water baths now offer interchangeable heating elements and temperature sensors, allowing researchers to tailor the device to their specific needs.
Modularity also extends to the integration of additional features, such as shaking mechanisms or circulatory pumps, which can be added as needed to enhance the functionality of the water bath. This flexibility allows laboratories to optimize their equipment for a wide range of applications, from simple temperature control to complex biochemical assays.
Advanced Materials and Coatings
The use of advanced materials and coatings is another significant trend in the development of electrolytic cell water baths. Traditional materials like stainless steel and glass are being replaced or supplemented with newer, more durable and chemically resistant materials. For example, some water baths now use high-performance polymers that can withstand harsh chemicals and extreme temperatures, ensuring long-term reliability and performance.
Additionally, advanced coatings are being applied to the interior surfaces of water baths to prevent corrosion and facilitate easy cleaning. These coatings can also improve heat transfer efficiency, further enhancing the overall performance of the device.
Conclusion
The future of electrolytic cell water baths is bright, with numerous innovations poised to transform this essential laboratory equipment. From the integration of smart technologies and energy-efficient designs to enhanced safety features and customizable solutions, these advancements promise to make water baths more precise, efficient, and adaptable to a wide range of research needs. As these trends continue to evolve, they will undoubtedly play a crucial role in advancing scientific research and development across various fields.
Related Products
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- Flat Corrosion Electrolytic Electrochemical Cell
Related Articles
- The Thermodynamic Paradox: Balancing Precision and Safety in Electrolytic Cells
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- Understanding Electrolytic Cells and Their Role in Copper Purification and Electroplating
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
























