Introduction to Electrolytic Cells in Coating Evaluation
In the realm of industrial applications, the evaluation of coatings plays a pivotal role in ensuring the durability, functionality, and aesthetics of various products. Electrolytic cells have emerged as a cornerstone in this process, offering a sophisticated means to assess and enhance coating properties. This article delves into the advanced techniques of coating evaluation using electrolytic cells, exploring the intricacies of electroplating, sol-gel methodologies, and wet chemical techniques. By unraveling the fundamentals and practical applications of these methods, we aim to equip readers with a deeper understanding of how electrolytic cells contribute to the selection and optimization of coating materials, ultimately leading to improved product performance and reliability.
Fundamentals of Electroplating for Coating Applications
Electroplating is a critical process in the field of metallurgy and materials science, utilized to deposit a thin layer of one metal onto the surface of another, typically non-metallic, material. This technique is employed to enhance the properties of the base material, such as improving its resistance to corrosion, enhancing its aesthetic appeal, or providing electrical conductivity. The process involves the use of an electrolytic cell, where the object to be plated (cathode) is immersed in a solution containing metal ions (electrolyte) and an electrical current is applied.
Mechanisms of Electroplating
The fundamental principle behind electroplating is Faraday's laws of electrolysis, which dictate that the amount of metal deposited is proportional to the amount of electricity passed through the solution. During the process, the anode, which is typically the metal to be deposited, dissolves into the electrolyte, releasing metal ions. These ions are then attracted to the cathode, where they deposit and form a coherent metal layer.

Controlling the Thickness of the Plated Layer
The thickness of the metal layer deposited on the cathode can be controlled by several parameters:
- Concentration of Metal Ions in the Electrolyte: A higher concentration leads to more ions being available for deposition, resulting in a thicker layer.
- Applied Current: Increasing the current increases the rate of ion deposition.
- Plating Time: Prolonging the duration of the process allows for more ions to be deposited.
Quality Requirements for the Coating Layer
The quality of the electroplated layer is crucial for its effectiveness. Key requirements include:
- Adherence: The coating must adhere tightly to the base material to prevent peeling or flaking.
- Uniformity: The layer should be uniform across the surface to ensure consistent properties.
- Density: A dense coating is less porous and provides better protection against environmental factors.
Electro-Typing
Another application of electroplating is electro-typing, used in the reproduction of typefaces and artwork. This process involves creating a mold of the original item in wax, coating it with a conductive material, and then subjecting it to electroplating to form a metal replica. This technique is invaluable in the preservation and replication of historical documents and artworks.
In conclusion, electroplating is a versatile and essential process that offers numerous benefits in terms of material enhancement and protection. By understanding and controlling the mechanisms and parameters of electroplating, industries can tailor the properties of their products to meet specific requirements, ensuring durability, functionality, and aesthetic appeal.
Sol-Gel Methodology in Electrolytic Coating
The sol-gel method represents a sophisticated and versatile approach to the deposition of thin films, particularly in electrolytic coatings. This method distinguishes itself from traditional wet chemical methods through its unique processing techniques and the superior properties of the films it produces. The sol-gel process involves the transformation of a liquid colloidal suspension, known as a "sol," into a solid network, or "gel," through a series of chemical reactions. This section delves into the intricacies of the sol-gel method, highlighting its advantages, key reactions, and considerations for effective application in electrolytic coatings.
Key Reactions in the Sol-Gel Process
The sol-gel process is characterized by three primary reactions: hydrolysis, alcohol condensation, and water condensation. These reactions are pivotal in shaping the final properties of the coating. Hydrolysis involves the reaction of the precursor with water, leading to the formation of hydroxyl groups. Subsequently, these hydroxyl groups undergo condensation reactions, either with other hydroxyl groups (water condensation) or with alcohol groups (alcohol condensation), to form a polymeric network.
The control of these reactions is crucial as it directly influences the structural and functional properties of the resulting gel. Factors such as pH, temperature, reaction time, reagent concentrations, and the nature and concentration of catalysts play significant roles in modulating the rate and extent of these reactions. By judiciously manipulating these parameters, it is possible to tailor the sol-gel process to achieve desired film characteristics, such as thickness, uniformity, and surface roughness.
Advantages of the Sol-Gel Method in Coating Applications
One of the most significant advantages of the sol-gel method is its ability to produce coatings at relatively low temperatures. This is particularly beneficial for sensitive materials that may degrade or undergo structural changes at higher temperatures. The low-temperature processing not only preserves the integrity of the substrate but also allows for the formation of compounds with good crystallinity and uniform particle size distribution, often in the nanoscale range.
Moreover, the sol-gel method offers excellent control over the stoichiometric ratio of the coating materials, ensuring precise composition and high-quality films. The simplicity of the fabrication process, coupled with its scalability, makes the sol-gel method an attractive option for both laboratory-scale experiments and large-scale industrial applications. Despite these advantages, the method is not without challenges, including issues related to low yield, high cost of precursors, and the potential for heterogeneous and discontinuous coating layers.
Application in Electrolytic Coatings
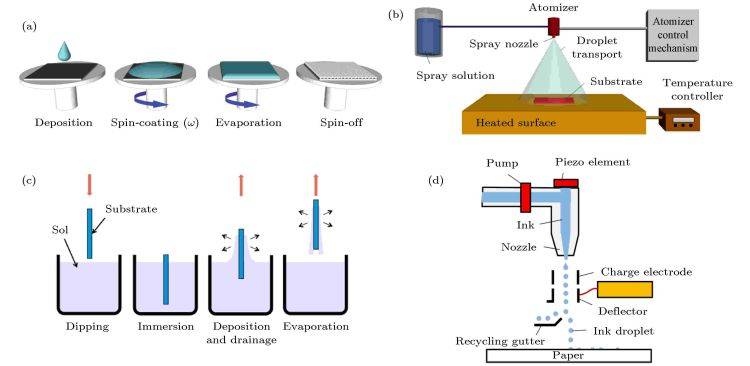
In the context of electrolytic coatings, the sol-gel method is particularly valued for its ability to cover surfaces of any size and shape, ensuring uniform coverage over vast areas. This is achieved through various deposition techniques such as spraying, dipping, or spinning, each tailored to meet specific application requirements. The sol-gel coatings are known for their good homogeneity and low surface roughness, which are critical for enhancing the performance and longevity of electrolytic devices.
Furthermore, the sol-gel method is conducive to the incorporation of various functional additives, such as catalysts or dopants, into the coating matrix. This capability allows for the creation of multifunctional coatings that can meet complex application demands, such as enhanced conductivity, improved mechanical strength, or resistance to environmental degradation.
Conclusion
In summary, the sol-gel method stands out as a powerful and flexible technique for the deposition of thin films in electrolytic coatings. Its ability to operate at low temperatures, combined with its excellent control over film properties and its scalability, positions it as a leading choice in both research and industrial settings. While challenges remain, ongoing advancements in sol-gel chemistry and process optimization continue to expand its potential and refine its application in the field of electrolytic coatings.
Wet Chemical Techniques for Coating Evaluation
Wet chemical techniques play a pivotal role in the evaluation and application of coatings, offering a diverse array of methods to achieve uniform and effective coatings. These techniques, which include hydrothermal/solvothermal methods and other wet chemical processes, are particularly effective in coating applications due to their ability to manipulate the chemical environment at the molecular level. This section delves into the specifics of these techniques, their effectiveness, and the challenges associated with achieving uniform coatings.
Hydrothermal and Solvothermal Methods
Hydrothermal and solvothermal methods involve the use of high-temperature and high-pressure aqueous or solvent-based systems to synthesize materials. These methods are particularly useful for the deposition of coatings due to their ability to control the nucleation and growth of particles, leading to the formation of uniform coatings. The solvothermal method, which uses organic solvents, allows for greater control over the chemical environment, making it suitable for delicate substrates or those requiring specific chemical interactions.
One of the key advantages of hydrothermal and solvothermal methods is their ability to produce coatings with high crystallinity and purity. This is achieved by maintaining precise control over the reaction conditions, such as temperature, pressure, and the concentration of reactants. For instance, studies have shown that adjusting the pH of the solution can significantly influence the morphology and thickness of the resulting coatings.
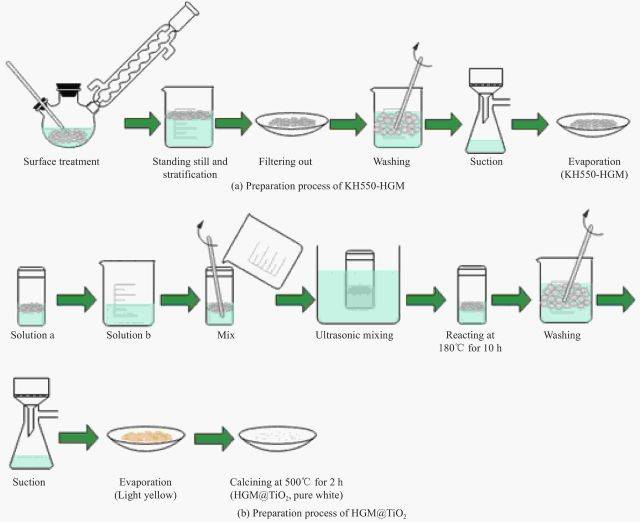
Other Wet Chemical Processes
Beyond hydrothermal and solvothermal methods, other wet chemical processes such as sol-gel, electrodeposition, and dip-coating are also extensively used in coating applications. These methods offer a range of benefits, including simplicity, cost-effectiveness, and the ability to produce coatings with excellent uniformity and adhesion.
Sol-Gel Process
The sol-gel process involves the conversion of a liquid "sol" into a solid "gel" through a series of chemical reactions. This method is particularly useful for producing coatings with tailored properties, such as optical transparency, thermal stability, and chemical resistance. The sol-gel process allows for the incorporation of various functional additives, such as nanoparticles or organic molecules, into the coating matrix, enhancing its overall performance.
Electrodeposition
Electrodeposition is a technique where a coating material is deposited onto a substrate from an electrolyte solution under the influence of an electric current. This method is widely used for depositing metals and alloys, offering precise control over the coating thickness and composition. Electrodeposition is particularly advantageous for applications requiring corrosion resistance and wear resistance, as it can produce dense and adherent coatings.
Dip-Coating
Dip-coating involves immersing a substrate into a coating solution and then withdrawing it at a controlled rate. This method is simple and versatile, making it suitable for a wide range of applications. Dip-coating allows for the production of uniform coatings with minimal surface roughness, which is crucial for applications requiring optical clarity or smooth surfaces.
Challenges in Achieving Uniform Coatings
Despite the advantages of wet chemical techniques, achieving uniform coatings remains a significant challenge. Factors such as substrate heterogeneity, solution stability, and process parameters can all influence the uniformity of the resulting coatings. For instance, variations in substrate surface roughness can lead to non-uniform coating thicknesses, while fluctuations in solution concentration can result in inconsistent coating properties.
Moreover, the scalability of wet chemical techniques is often limited, making it challenging to apply these methods to large-scale industrial production. The need for precise control over reaction conditions also necessitates sophisticated equipment and skilled personnel, adding to the overall cost of the process.
Conclusion
Wet chemical techniques offer a powerful toolkit for the evaluation and application of coatings, enabling the production of high-quality coatings with tailored properties. While these methods present several challenges, ongoing research and technological advancements continue to enhance their effectiveness and broaden their applicability. By carefully selecting and optimizing the appropriate wet chemical technique, it is possible to achieve uniform and durable coatings that meet the stringent requirements of various industrial and scientific applications.
Impact of Coating Properties on Analysis
Coatings play a crucial role in enhancing the performance and longevity of various materials across multiple industries. The selection of appropriate coating materials and methods is essential to achieve the desired properties, such as corrosion resistance, wear resistance, and thermal conductivity. Understanding the impact of coating properties on analysis is vital for ensuring the effectiveness and reliability of coated materials.
Thermal Conductivity
Thermal conductivity is a critical property of coatings, especially in applications where heat management is paramount. High thermal conductivity coatings are essential for dissipating heat efficiently, preventing thermal stress, and ensuring the stability of the coated material. For instance, in the electronics industry, thermal management coatings are used to prevent overheating of electronic components, thereby enhancing their performance and lifespan.
The thermal conductivity of a coating is influenced by its material composition and microstructure. Metals like copper and aluminum have high thermal conductivity, making them suitable for thermal management applications. Ceramic coatings, on the other hand, have lower thermal conductivity but offer excellent thermal insulation properties. The choice of coating material depends on the specific thermal requirements of the application.
Chemical Stability
Chemical stability is another critical factor in coating analysis. Coatings must withstand chemical reactions and environmental exposure without degrading or losing their protective properties. Chemical stability is particularly important in corrosive environments, where coatings must resist chemical attack from acids, bases, and salts.
Coatings made from materials such as stainless steel, titanium, and certain ceramics exhibit excellent chemical stability. These materials form a protective barrier that prevents the underlying substrate from reacting with corrosive substances. In addition, some coatings are designed to react with the environment, forming a passive layer that further enhances their chemical stability.
Mechanical Properties
The mechanical properties of coatings, such as hardness, toughness, and flexibility, significantly impact their performance and durability. Hard coatings provide excellent wear resistance, protecting the underlying substrate from abrasive forces. Tough coatings, on the other hand, offer good resistance to impact and deformation, making them suitable for applications where the coated material is subjected to mechanical stress.
Flexible coatings are essential for applications where the coated material undergoes repeated bending or stretching. These coatings prevent cracking and delamination, ensuring the long-term integrity of the coating. The mechanical properties of a coating are determined by its material composition, microstructure, and the deposition process used.
Coating Thickness
Coating thickness is a critical parameter that affects the performance and analysis of coated materials. Thicker coatings provide better protection and durability but may obscure underlying features during analysis. Conversely, thinner coatings may not provide adequate protection but allow for better visibility of the substrate's features.
The optimal coating thickness depends on the specific requirements of the application. For instance, in the aerospace industry, coatings must be thin enough to minimize weight while providing sufficient protection against corrosion and wear. In analytical applications, the coating thickness must be carefully controlled to ensure that the features of interest are not obscured.
Secondary Electron Yield
The secondary electron yield (SEY) is an important property of coatings in analytical applications. Coatings with high SEY enhance the detection of secondary electrons, providing better imaging and analysis of the coated material. Metals with high SEY, such as gold and platinum, are often used for coating samples in scanning electron microscopy (SEM) to improve the quality of the images.
Dissolvable Coatings
In some analytical applications, it is necessary to remove the coating after analysis. Dissolvable coatings made from materials like silver and copper can be easily dissolved using appropriate solvents, allowing for the examination of the underlying substrate. These coatings are particularly useful in applications where the coating must be removed without damaging the substrate.
Conclusion
The impact of coating properties on analysis is significant, and selecting the appropriate coating materials and methods is essential for achieving the desired performance and reliability. Thermal conductivity, chemical stability, mechanical properties, coating thickness, secondary electron yield, and dissolvable coatings are all critical factors that must be considered in the analysis of coated materials. By understanding these properties and their implications, analysts can ensure the effectiveness and accuracy of their evaluations, leading to improved performance and longevity of coated materials in various applications.
Coating Thickness and Its Role in Feature Visibility
The thickness of a coating plays a pivotal role in determining the visibility and durability of features of interest on various materials. This section delves into the optimal coating thickness for different applications, ensuring that the features remain discernible and the coating withstands the intended use.
Importance of Coating Thickness
Coating thickness is a critical parameter in physical vapor deposition (PVD) processes. A thicker coating generally offers greater durability and resistance to wear and tear. However, a balance must be struck to ensure that the coating does not obscure underlying features. For instance, in decorative applications with mild to moderate wear, coatings that are a few tenths of a micrometer (0.2 to 0.5μm) thick can endure many years of use without significant wear. Conversely, for products subjected to harsher conditions, a thicker coating (typically greater than 1μm) is necessary. Additionally, the substrate should be harder to support the coating, as a thin coating can deflect to its fracture point if the substrate yields under localized pressure.
Uniformity and Thickness Control
Uniformity in coating thickness is essential for maintaining consistent material characteristics and ensuring optimal performance of the end product. Non-uniform or uneven film thickness can lead to variations in the material’s properties, which can affect the product’s performance. Factors such as the deposition rate, temperature, and other process parameters must be meticulously managed to achieve uniformity and precise thickness control.
Adhesion and Delamination
The long-term reliability and functionality of a coated product depend on the proper adhesion between the thin film and the substrate. Delamination, where the thin layer separates from the substrate, can result in product failure. The deposition technique, substrate preparation, and interfacial treatments are all critical elements that influence adhesion. Ensuring robust adhesion is crucial to prevent delamination and maintain the integrity of the coating.

Impact on Feature Visibility
The physical properties of the coating material, such as its thermal conductivity and brittleness, can impact the analysis of features of interest. Brittle metals, for example, can develop cracks when pressure is applied shortly after coating, hindering the visibility of features. Chemical stability is also vital, as some coatings may need to be removed after analysis. Metals like silver and copper are suitable options due to their ease of dissolution.
Adjusting Coating Thickness
The thickness of the coating should be tailored to the specific features of interest to prevent obscuration. For instance, in applications where the secondary electron yield is crucial, coating samples with a metal that has the highest secondary electron yield is ideal. The coating’s thickness must be adjusted to ensure that the features remain visible and accessible for analysis.
In conclusion, the optimal coating thickness varies depending on the application and the specific requirements of the features of interest. By carefully considering factors such as durability, uniformity, adhesion, and visibility, one can select the appropriate coating thickness to ensure that the features remain discernible and the coating performs as intended.
Case Studies: Successful Applications of Electrolytic Coating
Electrolytic coating techniques have revolutionized various industries by providing durable, functional, and aesthetically pleasing coatings. This section explores several real-world applications where electrolytic coatings have been successfully implemented, highlighting the outcomes and lessons learned.
Aerospace Industry
In the aerospace sector, the demand for lightweight yet robust materials is paramount. Electrolytic coatings have been instrumental in enhancing the performance and longevity of aircraft components. For instance, aluminum alloys, which are commonly used in aircraft construction, are often coated with anodic films to improve their corrosion resistance and wear characteristics. These anodic films are produced through a process called anodizing, where the aluminum parts are immersed in an acid electrolyte and subjected to an electric current. This process forms a hard, protective oxide layer on the aluminum surface, which can be further sealed to enhance its durability.
A notable case study involves the use of hard anodizing on landing gear components. By applying a thick anodic coating, the service life of these critical components has been extended by up to 50%, significantly reducing maintenance costs and downtime. Additionally, the anodized surfaces exhibit excellent thermal conductivity, which helps dissipate heat generated during high-speed flights, thereby enhancing the overall safety and efficiency of the aircraft.
Automotive Industry
The automotive industry has also benefited immensely from electrolytic coatings. One of the most common applications is electroplating, where metal parts are coated with a thin layer of another metal, such as chromium, nickel, or gold. This process not only enhances the aesthetic appeal of the vehicle but also provides essential protection against corrosion and wear.
For example, in the production of automotive trim, electroplated chromium coatings are widely used to achieve a glossy, mirror-like finish. These coatings are not only visually appealing but also highly resistant to corrosion, ensuring that the trim remains intact even in harsh environmental conditions. Furthermore, electroplated coatings on engine components, such as pistons and valves, improve their wear resistance, thereby enhancing the overall performance and reliability of the engine.
Electronics Industry
In the electronics industry, electrolytic coatings play a crucial role in protecting sensitive components from environmental factors and ensuring their optimal functionality. One notable application is the use of gold electroplating on connectors and contacts. Gold is an excellent conductor of electricity and is highly resistant to corrosion, making it ideal for these applications.
A case study involving the production of printed circuit boards (PCBs) illustrates the effectiveness of gold electroplating. By applying a thin layer of gold to the copper traces on the PCB, the reliability and lifespan of the board are significantly increased. This is particularly important in high-precision applications, such as smartphones and medical devices, where even minor corrosion can lead to significant performance issues.
Biomedical Industry
The biomedical industry has also embraced electrolytic coatings to enhance the functionality and biocompatibility of medical devices. One prominent application is the use of titanium anodizing in the production of implants. Titanium is a preferred material for implants due to its excellent biocompatibility and mechanical properties. However, to further improve its integration with the human body, titanium surfaces are often anodized to create a porous oxide layer.
This porous layer not only enhances the surface area of the implant but also promotes osseointegration, the process by which bone tissue grows into the porous structure of the implant. A notable case study involves the use of anodized titanium implants in dental applications. These implants have shown superior osseointegration rates compared to traditional implants, leading to faster healing times and improved patient outcomes.
Conclusion
The successful applications of electrolytic coatings across various industries underscore their versatility and effectiveness. From enhancing the performance of aerospace components to improving the reliability of electronic devices, electrolytic coatings have proven to be a valuable technology. As research and development continue to advance, we can expect even more innovative applications of electrolytic coatings, further driving progress in numerous fields.
Future Trends and Innovations in Electrolytic Coating
The field of electrolytic coating is poised for significant advancements and innovations that will shape its future applications and efficiencies. As industries continue to seek more sustainable, efficient, and high-performance coating solutions, several key trends and technologies are emerging. This section delves into these developments, focusing on potential improvements and new applications within the realm of electrolytic coating.
Atomic Layer Deposition (ALD)
Atomic Layer Deposition (ALD) represents a cutting-edge technique in the field of electrolytic coating, particularly for enhancing the surface properties of cathode materials in batteries. ALD involves the sequential use of two or more precursor chemicals to form a thin, uniform layer on a substrate. This method leverages a self-limiting chemical reaction, ensuring precise control over the thickness and uniformity of the deposited film. The advantages of ALD include its ability to coat complex geometries uniformly, its applicability to a wide range of materials, and its relatively low operational temperatures. However, the technique is currently limited by its complexity and the high costs associated with the necessary equipment and precursor materials.
Nanostructured Coatings and Nanocomposites
The production of nanostructured coatings and nanocomposites is another significant trend in electrolytic coating. These materials offer enhanced properties such as increased strength, improved electrical conductivity, and better resistance to environmental degradation. The development of these nanostructured materials is driven by the need for more efficient and durable coatings in applications ranging from electronics to renewable energy systems.
Ecological Considerations
Ecological sustainability is a critical driver in the evolution of electrolytic coating technologies. Innovations aimed at reducing effluent output and power consumption are paramount. For instance, advancements in coating processes that minimize waste and energy usage are being explored. These efforts not only contribute to environmental conservation but also align with global initiatives to reduce industrial carbon footprints.
Improved Functionality and New Applications
The electrolytic coating industry is also witnessing improvements in the functionality of existing products and the creation of entirely new applications. For example, the electronics industry requires high-purity materials for semiconductor devices and integrated circuits. Sputtering targets used in this industry produce conductive and dielectric thin films, which are essential for the performance and miniaturization of electronic components.
In the solar energy sector, the development of thin-film solar cells, such as those made from copper indium gallium selenide (CIGS), has been facilitated by advanced coating techniques. These cells represent the third generation of solar technology and are known for their efficiency and cost-effectiveness. The sputtering coating process is particularly favored for its ability to produce high-quality, uniform films.
Technological Synergies and Combinations
Another trend in electrolytic coating is the combination of different deposition techniques to leverage their respective strengths. For instance, combining physical vapor deposition (PVD) with chemical vapor deposition (CVD) can enhance the durability, friction reduction, and thermal properties of coatings. This synergistic approach allows for the creation of multi-layered coatings that offer superior performance characteristics.
Mathematical Modeling and Simulation
Advancements in mathematical modeling and numerical simulation are playing a crucial role in optimizing electrolytic coating processes. These tools help in understanding and predicting the behavior of coating systems, leading to improvements in reactor design and operational efficiencies. Such advancements are expected to reduce costs and enhance the mechanical properties of the films, making them more robust and reliable.
In conclusion, the future of electrolytic coating is bright with numerous innovations and trends that promise to enhance its efficiency, sustainability, and applicability across various industries. As research and development continue, these advancements will undoubtedly lead to the creation of more advanced, durable, and environmentally friendly coating solutions.
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Platinum Auxiliary Electrode for Laboratory Use
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Related Articles
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Vessel of Truth: Why the Container Matters More Than the Chemistry
- Handheld Coating Thickness Gauges: Accurate Measurement for Electroplating and Industrial Coatings