
Electrochemical Consumables
Rotating Platinum Disk Electrode for Electrochemical Applications
Item Number : ELEP
Price varies based on specs and customizations
$49.90 / set
- Specifications
- 0.5 ~ 6mm, can be customized
- Applicable temperature range
- 0 ~ 60℃
- Rod Material
- PTFE
- Guide material
- high Purity Platinum> 99.99%
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
Introduction
Disk electrodes are essential components for electrochemical experiments, commonly used in three-electrode setups. They are circular with a small ring around the edge and can be micromachined to a very small size. Disk electrodes are often used for cost-efficient disposable electrodes and are key applications for electrochemical techniques like cyclic voltammetry and impedance spectroscopy.
Technical specifications

| Specifications | 0.5 ~ 6mm, can be customized |
| Applicable temperature range | 0 ~ 60℃ |
| Rod Material | PTFE |
| Guide material | high Purity Platinum> 99.99% |
Detail & Parts
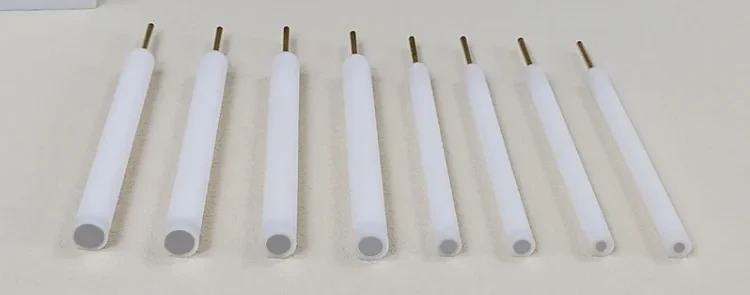

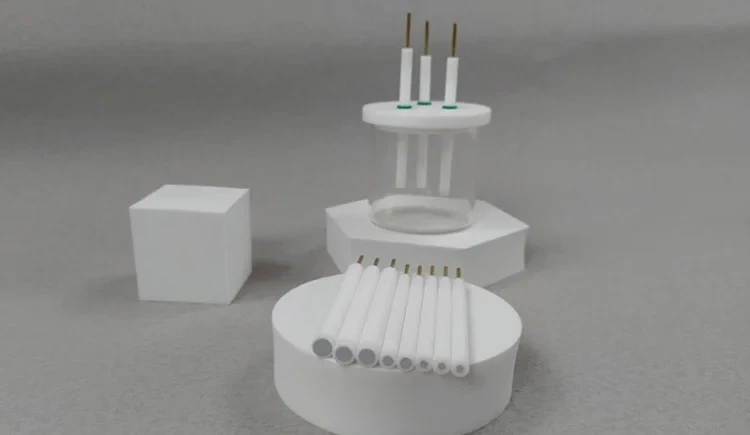
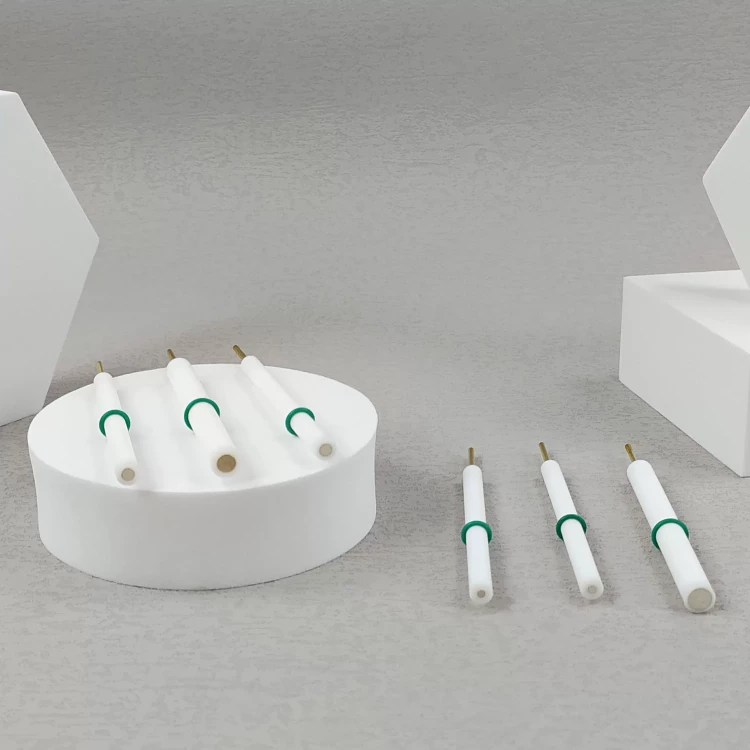

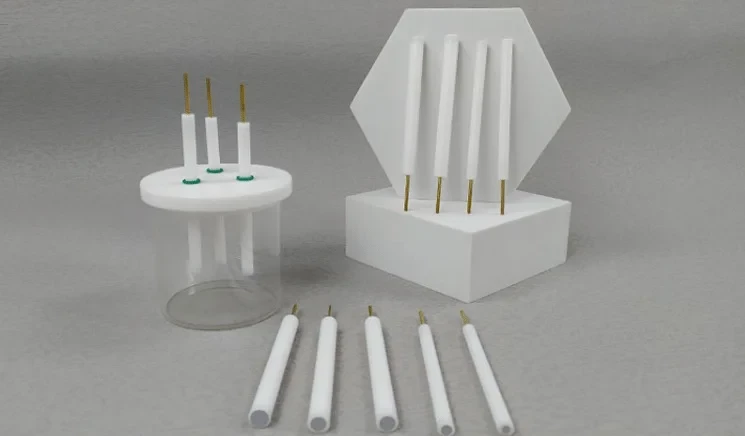

Applications
Platinum Disc Electrodes are widely used in various electrochemical techniques for substrate analysis. Here are some key application areas:
- Electrochemical analysis: Disk electrodes are used in a variety of electrochemical analysis techniques, such as cyclic voltammetry, chronoamperometry, and impedance spectroscopy. These techniques can be used to study the electrochemical properties of materials, such as their redox behavior, conductivity, and capacitance.
- Electrocatalytic measurements: Platinum Disc Electrodes are commonly used as the disk electrode in rotating ring-disk electrodes (RRDEs) for electrocatalytic measurements. Due to its low electrocatalytic activity compared to Pt and Au, glassy carbon (GC) is often used as the disk electrode onto which the electrocatalyst is deposited.
- Direct dropping experiments: Very small Platinum Disc Electrodes can be used for direct dropping experiments, where a small drop of a solution of interest is put directly onto the electrode and the electrical impedance is measured.
- Fuel cells: Platinum thin films are crucial in fuel cells, providing high electro-catalytic property with low resistance.
- Solar cells: Platinum counter electrodes are used in solar cells made of dye-synthesized TiO2, improving the ionic diffusion rate and enabling high current draw.
- Gas electrodes and polymers: Platinum is deposited on various substrates like gas electrodes and polymers through impregnation and reduction methods. The sputtering technique allows for precise distribution of metal and homogenous particles with controlled thickness.
Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Is An Electrode In Electrochemistry?
What Is Rotating Disk Electrode Used For?
What Is The Function Of Auxiliary Electrode?
What Are The Materials Used In Electrochemical Cell?
What Are The 3 Electrodes In Electrochemistry?
What Is The Rotating Electrode Method?
What Is The Difference Between Auxiliary And Reference Electrode?
What Are The Examples Of Electrochemical Material?
What Are The Different Types Of Electrochemical Electrodes?
What Is The Rotating Ring-disk Electrode Method?
What Materials Are Commonly Used For Auxiliary Electrodes?
What Materials Are Commonly Used For Electrochemical Electrodes?
What Are The Advantages Of Rotating Disc Electrode?
How Do Auxiliary Electrodes Affect The Performance Of An Electrochemical Cell?
What Factors Should Be Considered When Selecting An Electrochemical Electrode?
Why Are Auxiliary Electrodes Necessary In Electrochemical Systems?
How Can Electrochemical Electrodes Be Used In Various Applications?
Are There Any Limitations Or Considerations When Using Auxiliary Electrodes?
4.8 / 5
Platinum disc electrode is a great product, definitely worth the price. It's super durable and easy to use, definitely recommend!
4.9 / 5
The technology behind this electrode is amazing. I was able to get accurate and consistent results with ease.
4.7 / 5
The quality of the electrode is top-notch. The platinum coating is thick and evenly distributed, resulting in consistent and reliable measurements.
4.8 / 5
The delivery was super fast, I received my order within 2 days. The packaging was also very secure, ensuring the product's safety during transit.
4.6 / 5
This electrode is a game-changer. It has helped me obtain more precise data in my electrochemical experiments.
4.9 / 5
The electrode's performance exceeded my expectations. It's a great value for money, and I highly recommend it to anyone in the field of electrochemistry.
4.8 / 5
The customer service is excellent. I had a minor issue with my order, and they resolved it promptly and efficiently.
4.7 / 5
The durability of this electrode is impressive. I've been using it for months, and it still performs as well as when I first got it.
4.9 / 5
The technological advancement of this electrode is remarkable. It incorporates cutting-edge features that make it stand out in the market.
4.8 / 5
The electrode's design is well-thought-out, making it user-friendly and efficient for various electrochemical applications.
4.7 / 5
The electrode's compatibility with different electrochemical instruments is a huge plus, allowing for seamless integration into existing setups.
4.9 / 5
The electrode's versatility makes it suitable for a wide range of experiments, catering to diverse research needs.
4.8 / 5
The electrode's accuracy and precision are commendable, providing reliable and reproducible data for analysis.
4.7 / 5
The electrode's durability is exceptional, withstanding repeated use and harsh experimental conditions without compromising performance.
4.9 / 5
The electrode's affordability makes it accessible to researchers with limited budgets, promoting wider adoption and scientific advancements.
4.8 / 5
The electrode's compact design and portability make it convenient for use in various laboratory settings, facilitating research on the go.
4.7 / 5
The electrode's ease of maintenance and cleaning minimizes downtime, maximizing productivity and efficiency in the laboratory.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Elevate your electrochemical research with our Rotating Disk and Ring Electrodes. Corrosion resistant and customizable to your specific needs, with complete specifications.

Platinum Sheet Electrode for Laboratory and Industrial Applications
Elevate your experiments with our Platinum Sheet Electrode. Crafted with quality materials, our safe and durable models can be tailored to fit your needs.

Platinum Sheet Electrode for Battery Lab Applications
Platinum sheet is composed of platinum, which is also one of the refractory metals. It is soft and can be forged, rolled and drawn into rod, wire, plate, tube and wire.

Electrode Polishing Material for Electrochemical Experiments
Looking for a way to polish your electrodes for electrochemical experiments? Our polishing materials are here to help! Follow our easy instructions for best results.

Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
High-quality graphite electrodes for electrochemical experiments. Complete models with acid and alkali resistance, safety, durability, and customization options.

Gold Electrochemical Sheet Electrode Gold Electrode
Discover high-quality gold sheet electrodes for safe and durable electrochemical experiments. Choose from complete models or customize to meet your specific needs.

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.

Lab Electrochemical Workstation Potentiostat for Laboratory Use
Electrochemical workstations, also known as laboratory electrochemical analyzers, are sophisticated instruments designed for precise monitoring and control in various scientific and industrial processes.

Copper Sulfate Reference Electrode for Laboratory Use
Looking for a Copper Sulfate Reference Electrode? Our complete models are made of high-quality materials, ensuring durability and safety. Customization options available.

Flat Corrosion Electrolytic Electrochemical Cell
Discover our flat corrosion electrolytic cell for electrochemical experiments. With exceptional corrosion resistance and complete specifications, our cell guarantees optimal performance. Our high-quality materials and good sealing ensure a safe and durable product, and customization options are available.

Electrolytic Electrochemical Cell for Coating Evaluation
Looking for corrosion-resistant coating evaluation electrolytic cells for electrochemical experiments? Our cells boast complete specifications, good sealing, high-quality materials, safety, and durability. Plus, they're easily customizable to meet your needs.

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.
Related Articles

Exploring Rotating Electrode Technology in Electrochemistry
An in-depth analysis of rotating electrode technology, its applications, and its impact on electrochemical reactions under different flow conditions.

Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
Explore the detailed workings, applications, and significance of Rotating Disk Electrodes (RDE) in electrochemical research. Discover how RDEs are used in fuel cells, catalyst development, and more.

Electrode Materials for Rotating Ring-Disk Electrodes
Rotating ring-disk electrodes (RRDEs) are used in a wide range of applications, from fuel cells to sensors, and they require careful selection of electrode materials for optimal performance.

Electrochemical Electrodes in Chemical Analysis
Electrochemical electrodes are essential tools used in many chemical analysis techniques and experiments. These electrodes are devices that allow us to measure the electrical potential difference in a chemical reaction.

Understanding Electrodeposition with Electrochemical Electrodes
Electrodeposition is a process of depositing a metal or a non-metallic material onto a surface by applying an electric current.

Electrolytes and Electrochemical Electrodes
Electrolytes and electrodes play an essential role in electrochemistry. Electrolytes are substances that conduct electricity when dissolved in water or melted.

Innovations in Electrochemical Electrodes Technology
Recent advancements in nanotechnology and materials science have led to significant improvements in electrochemical devices, making them more efficient, durable, and cost-effective.

Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
Explore the world of reference electrodes with our detailed guide. Learn about different types, their applications, and how to select the right one for your needs. Ideal for researchers and lab technicians.

A Comprehensive Guide to Reference Electrodes
Reference electrodes are used in electrochemical measurements to establish a stable potential against which the potential of the working electrode can be measured.

The Future of Electrochemical Electrodes
The latest trends and developments in electrode materials and their implications for the future of electrochemistry.

A Beginner's Guide to Understanding Reference Electrodes in Electrochemistry
Reference electrodes provide a stable and known potential that other electrodes can be compared to, allowing for accurate measurements of electrochemical reactions.

How to Choose the Right Electrochemical Electrode
The choice of electrode material can have a significant impact on the performance of the electrochemical system.