Introduction to Rotating Disk Electrode (RDE)
The Rotating Disk Electrode (RDE) stands as a pivotal tool in the realm of electrochemical studies, offering precise control over mass transport and significantly enhancing the efficiency of electrochemical reactions. This comprehensive guide delves into the intricacies of RDE technology, exploring its fundamental structure, hydrodynamic properties, and the pivotal role it plays in various experimental setups. By understanding how rotation affects the flux of analytes and the principles of diffusion layer control, researchers can harness the full potential of RDE in applications ranging from fuel cell research to catalyst development. Join us as we unravel the complexities of RDE and its transformative impact on electrochemical research.
Fundamentals of RDE Technology
The Rotating Disk Electrode (RDE) is a critical tool in electrochemical research, particularly in the study of mass transport and reaction kinetics at electrode surfaces. This technology leverages the hydrodynamic properties of a rotating disk to control the diffusion layer and enhance the precision of electrochemical measurements.
Hydrodynamic Properties of RDE
The RDE consists of a disk electrode, typically made from materials such as platinum, gold, or glassy carbon, embedded in an insulating surround, often made of PTFE. The electrode is rotated about its vertical axis at speeds ranging from 400 to 10,000 revolutions per minute (rpm). This rotation induces a laminar flow of the electrolyte solution, which significantly affects the mass transport of analytes to the electrode surface.
The hydrodynamic theory of the RDE assumes uniform accessibility to the electrode surface, which allows for precise and reproducible control over convection and diffusion. This uniformity is crucial for studying the kinetics of interfacial processes, making the RDE an invaluable tool in fields such as corrosion studies, fuel cell research, and catalyst development.

Rotation and Flux of Analytes
The rotation of the disk electrode creates a constant flux of analytes to the electrode surface. This is achieved through the generation of a convective flow that brings reactants from the bulk solution to the electrode surface, where they can undergo electrochemical reactions. The rate of rotation directly influences the thickness of the diffusion layer, which in turn affects the rate of mass transport.
Higher rotation speeds result in thinner diffusion layers, leading to increased mass transport rates. Conversely, lower rotation speeds produce thicker diffusion layers, which can be advantageous for studying slower reaction kinetics. The ability to control the diffusion layer thickness through rotation speed provides a powerful means of manipulating the electrochemical environment.
Principles of Diffusion Layer Control
Controlling the diffusion layer is essential for accurate electrochemical measurements. The RDE achieves this through its hydrodynamic design, which ensures that the solution flow is laminar and well-defined. Laminar flow minimizes turbulence, allowing for a more predictable and controlled mass transport of reactants to the electrode surface.
The diffusion layer thickness can be mathematically modeled and experimentally controlled by adjusting the rotation speed. This control is crucial for experiments that require precise quantification of reaction rates and kinetics. The RDE's ability to maintain a steady-state mass transport over a wide range of conditions makes it a versatile tool in electrochemical research.
Flow Dynamics and Mass Transport Mechanisms
The flow dynamics at the RDE are characterized by the movement of the electrolyte solution around the rotating disk. As the disk rotates, it creates a centrifugal force that drives the solution outward from the center of the disk. This movement induces a convective flow that brings reactants from the bulk solution to the electrode surface.
The mass transport mechanisms at the RDE involve both convection and diffusion. Convection is the primary mechanism for transporting reactants to the electrode surface, while diffusion ensures the distribution of reactants within the diffusion layer. The interplay between these two mechanisms determines the overall mass transport rate and the resulting electrochemical response.
Advantages and Drawbacks of RDE
The RDE offers several advantages, including rapid establishment of a steady-state mass transport and easily reproducible control over convection. These features make it an ideal tool for studying reaction kinetics and mass transport phenomena. However, there are also drawbacks, such as the complexity of electrode and cell construction and the need for theoretical treatments to determine solution flow velocity profiles.
In conclusion, the Rotating Disk Electrode is a fundamental technology in electrochemical research, providing precise control over hydrodynamic properties and mass transport mechanisms. Its ability to manipulate the diffusion layer and enhance the precision of electrochemical measurements makes it an indispensable tool for advancing our understanding of interfacial processes and reaction kinetics.
Experimental Setup and Operation of RDE
The Rotating Disk Electrode (RDE) is a critical component in electrochemical studies, particularly in a three-electrode system where it serves as the working electrode. This setup allows for precise control and determination of reactant transportation near the electrode surface, which significantly impacts the electrode reaction mechanism and kinetics. The RDE is pivotal in investigating various phenomena, including redox chemistry, and is widely used in applications such as fuel cells, hydrogen production, depollution, and electrochemical sensing.
Selection of Materials
The RDE consists of a conductive disk, typically made from noble metals like platinum or gold, glassy carbon, or other conductive materials based on specific experimental needs. The disk is embedded in an inert, non-conductive polymer or resin, such as PTFE (Polytetrafluoroethylene), to ensure stability and prevent electrical interference. The choice of material for the disk is crucial as it affects the electrode's conductivity, durability, and reactivity with the analytes.
Assembly of the Three-Electrode System
The three-electrode system in RDE experiments includes the working electrode (RDE), a reference electrode, and a counter electrode. The reference electrode provides a stable potential reference point, while the counter electrode balances the current flow. The assembly process involves careful alignment and connection of these electrodes to the potentiostat, which controls the electrical parameters of the experiment. Proper assembly ensures accurate data collection and minimizes experimental variability.

Calibration of the Rotating Mechanism
The rotation speed of the RDE is a critical parameter that directly influences the experimental outcomes. The electrode is attached to an electric motor with fine control over the rotation rate, typically varying between 400 and 10,000 rpm. Calibration of the rotating mechanism involves setting the desired rotation speed and ensuring stability and reproducibility. This step is essential for maintaining consistent hydrodynamic conditions and accurate measurement of mass transport-limited currents.
Importance of Rotation Speed Control
Control of the rotation speed is paramount in RDE experiments as it affects the convective and diffusive transport of reactants to the electrode surface. Higher rotation speeds enhance the mass transport rate, leading to more efficient utilization of the reactants and better kinetic data. Conversely, lower rotation speeds allow for detailed studies of slow kinetic processes. The precise control of rotation speed enables researchers to tailor the experimental conditions to specific research objectives, ensuring high-quality data and meaningful insights into the reaction mechanisms.
Experimental Outcomes and Applications
The RDE setup allows for a wide range of electrochemical techniques, including linear sweep voltammetry, cyclic voltammetry, and more complex methods like the Rotating Ring-Disk Electrode (RRDE) technique. These methods are invaluable for studying multi-electron processes, kinetics of slow electron transfer, adsorption/desorption steps, and electrochemical reaction mechanisms. The RDE's ability to provide precise control over hydrodynamic conditions makes it an essential tool in various fields, from fundamental electrochemistry to applied research in energy conversion and environmental science.
In summary, the experimental setup and operation of the RDE involve meticulous selection of materials, careful assembly of the three-electrode system, precise calibration of the rotating mechanism, and strategic control of rotation speed. These steps ensure accurate and reproducible data, enabling comprehensive studies of electrochemical processes and their applications in diverse scientific and technological domains.
Voltammetry Techniques with RDE
Voltammetry techniques that utilize Rotating Disk Electrode (RDE) are powerful tools in the study of redox reactions and other chemical phenomena. These techniques, including linear sweep voltammetry and cyclic voltammetry, offer unique insights into the kinetics and mechanisms of electrochemical processes.
Linear Sweep Voltammetry with RDE
Linear sweep voltammetry (LSV) involves sweeping the potential of the working electrode linearly with time while measuring the resulting current. When performed with an RDE, the rotation of the disk electrode enhances mass transport, leading to more efficient and controlled reactions. This setup allows for the investigation of various electrochemical phenomena, such as multi-electron transfer processes, the kinetics of slow electron transfers, and the adsorption/desorption steps.
By varying the rotation rates during LSV experiments, researchers can modulate the mass transport conditions and gain deeper insights into the reaction mechanisms. The enhanced mass transport at the RDE results in higher limiting currents compared to stationary electrodes, making it easier to detect and analyze minor reaction components.
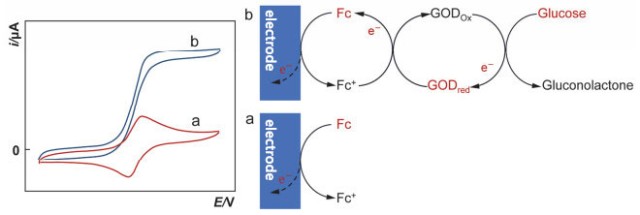
Cyclic Voltammetry with RDE
Cyclic voltammetry (CV) is another widely used technique that involves scanning the potential of the working electrode back and forth between two predetermined values. The resulting current-potential curve provides valuable information about the redox potentials, reaction kinetics, and mechanisms of the analyte.
When performed with an RDE, cyclic voltammetry exhibits distinct characteristics. The products of the potential sweep are continually swept away from the electrode, leading to a different behavior compared to stationary electrodes. The reversal of the potential sweep in CV produces an i-E curve that closely matches the forward scan, except for the capacitive charging current. This unique behavior makes RDE-CV suitable for studying the reactivity of electrode reaction products.
The peak current in a cyclic voltammogram for an RDE is typically a plateau-like region, governed by the Levich equation. The limiting current is significantly higher than that of a stationary electrode, as the mass transport of reactants is actively stimulated by the rotating disk. This enhanced mass transport allows for more accurate and sensitive measurements, making RDE-CV a valuable tool in electrochemical research.
Applications of RDE in Electrochemical Studies
The RDE setup allows for a wide range of experiments beyond the capabilities of stationary electrodes. For instance, while one electrode conducts linear sweep voltammetry, the other can be kept at a constant potential or swept in a controlled manner. This flexibility enables the study of multi-electron processes, the kinetics of slow electron transfer, adsorption/desorption steps, and electrochemical reaction mechanisms.
Moreover, the disk electrode can be immersed in solution and used for various electrochemical measurements. By performing techniques like cyclic voltammetry, researchers can learn about the redox potentials of substances and evaluate their suitability for inclusion in electronic devices.

Conclusion
Voltammetry techniques with RDE offer a powerful and versatile approach to studying redox reactions and other chemical phenomena. The enhanced mass transport and controlled reaction conditions provided by the RDE make these techniques invaluable in electrochemical research. By leveraging the unique capabilities of RDE, researchers can gain deeper insights into the kinetics and mechanisms of electrochemical processes, paving the way for advancements in various scientific and technological fields.
Applications of RDE in Electrochemical Research
The Rotating Disk Electrode (RDE) is a critical tool in electrochemical research, providing a controlled environment for studying various chemical reactions under hydrodynamic conditions. This technique is particularly useful in fields such as fuel cell research, corrosion studies, and catalyst development, offering insights into reaction mechanisms and kinetics.
Fuel Cell Research
In the realm of fuel cell research, RDE plays a pivotal role in understanding and optimizing the electrochemical reactions that occur within these devices. For instance, in proton exchange membrane (PEM) fuel cells, the reduction of dioxygen at the cathode is often catalyzed by platinum nanoparticles. The RDE allows researchers to study the efficiency and selectivity of these catalysts, particularly focusing on the reduction of oxygen to water and the minimization of by-products like hydrogen peroxide, which can be detrimental to the fuel cell's performance and longevity.
Recent advancements in RDE technology have enabled more precise measurements of catalyst activity and stability. For example, the Rotating Ring Disk Electrode (RRDE) configuration enhances the capabilities of RDE by allowing simultaneous detection of both the catalyst's primary reaction products and any secondary reactions that might occur. This dual-detection capability is crucial for fully understanding the electrocatalytic processes in fuel cells.
Corrosion Studies
RDE is extensively used in corrosion studies to investigate the electrochemical behavior of materials in different environments. By controlling the mass transport of reactants to the electrode surface, researchers can simulate various corrosion conditions and analyze the rate and mechanism of corrosion processes. This information is vital for developing strategies to prevent or mitigate corrosion, which is a significant concern in industries such as aerospace, automotive, and marine engineering.
Catalyst Development
In the field of catalyst development, RDE provides a robust platform for evaluating the performance of new and existing catalysts. The ability to control the flow of reactants to the electrode surface allows for detailed studies of catalyst activity, selectivity, and stability. This is particularly important in the development of catalysts for environmental applications, such as the reduction of pollutants or the production of hydrogen for energy storage.

For instance, RDE experiments can be used to assess the efficiency of catalysts in the electrochemical reduction of carbon dioxide to valuable chemicals and fuels. This process not only offers a potential solution to carbon capture but also contributes to the development of sustainable energy systems.
Case Studies and Recent Advancements
Recent studies have highlighted the versatility and effectiveness of RDE in various applications. For example, a study on the electrochemical reduction of nitrate ions using RDE demonstrated the potential of this technique in wastewater treatment, showcasing its applicability in environmental remediation.
Another notable advancement is the use of RDE in the development of novel materials for energy storage devices, such as supercapacitors and lithium-ion batteries. By studying the electrochemical behavior of these materials under controlled conditions, researchers can optimize their properties for improved energy storage and discharge capabilities.
In conclusion, the Rotating Disk Electrode is a versatile and powerful tool in electrochemical research, enabling detailed studies of reaction mechanisms and kinetics in a wide range of applications. From fuel cell research and corrosion studies to catalyst development, the RDE continues to drive advancements in science and technology, contributing to the development of sustainable and efficient energy solutions.
Advantages and Limitations of RDE
The Rotating Disk Electrode (RDE) is a pivotal tool in electrochemical research, offering enhanced control over mass transport and improved reaction kinetics. This section delves into the advantages of using RDE in electrochemical experiments, as well as the limitations and challenges associated with its technology.
Advantages of RDE
Enhanced Mass Transport Control: One of the primary advantages of the RDE is its ability to control mass transport through rotation. By varying the rotation speed, researchers can manipulate the flux of analyte to the electrode, thereby controlling the diffusion layer thickness. This is particularly useful in studies involving oxygen reduction reactions (ORR) and hydrogen evolution reactions (HER), where the rotation helps in quickly removing gas bubbles from the catalyst surface, ensuring a clean and active surface for the reaction.
Improved Reaction Kinetics: The hydrodynamic nature of the RDE allows for a more uniform distribution of reactants at the electrode surface, leading to improved reaction kinetics. This is crucial for studying the interfacial reactions of most electrochemical processes, including multi-electron processes, slow electron transfer kinetics, and adsorption/desorption steps. The controlled environment provided by the RDE enables more accurate and reproducible measurements of reaction rates and mechanisms.
Versatility in Experimental Setup: The RDE can be used in a three-electrode system, allowing for a wide range of electrochemical techniques such as linear sweep voltammetry, step experiments, and controlled potential sweeps. This versatility makes the RDE a valuable tool for both fundamental research and applied studies, catering to the specific needs of different systems.
Limitations and Challenges of RDE
Complexity in Electrode and Cell Construction: Despite its advantages, the construction of electrodes and cells for RDE experiments can be complex. The design must accommodate the rotating mechanism while ensuring electrical connectivity and mechanical stability. This complexity can pose challenges in terms of fabrication and maintenance, potentially limiting the accessibility of RDE technology to some researchers.
Theoretical Treatment and Data Analysis: The theoretical treatment of RDE experiments requires a detailed understanding of fluid dynamics, including the solution flow velocity profiles as functions of rotation rate, viscosities, and densities. This complexity necessitates sophisticated computational tools and a high level of expertise in data analysis. While there are well-established models and simulations available, the learning curve can be steep for newcomers to the field.
Alternative Controlled Flow Methods: While the RDE is a classical technique, there are alternative methods for controlled flow, such as the channel flow cell and the wall pipe and wall jet configurations. These methods offer their own set of advantages, including rapid establishment of steady-state mass transport and easily controlled convection over a wide range of mass transfer coefficients. However, they also come with their own set of drawbacks, such as the difficulty in constructing electrodes and cells, and the need for detailed theoretical treatment.
In conclusion, the Rotating Disk Electrode (RDE) offers significant advantages in terms of mass transport control and improved reaction kinetics, making it an invaluable tool in electrochemical research. However, the technology also comes with challenges related to complexity in construction, theoretical treatment, and the availability of alternative methods. Understanding these advantages and limitations is crucial for researchers to make informed decisions about the appropriate use of RDE in their experiments.
Comparison with Other Electroanalytical Techniques
The Rotating Disk Electrode (RDE) is a fundamental hydrodynamic technique in electroanalytical chemistry, providing a controlled environment for studying reaction mechanisms and kinetics. However, it is essential to consider other hydrodynamic and controlled flow methods, such as channel flow cells and wall jet configurations, to determine the most suitable technique based on specific experimental requirements and objectives.

Channel Flow Cells
Channel flow cells involve a continuous flow of electrolyte through a narrow channel, with the working electrode positioned within this channel. This method offers several advantages:
- High Mass Transport Rates: The continuous flow ensures rapid and steady-state mass transport, which is crucial for studying fast reactions.
- Reproducibility: The flow rate can be precisely controlled, leading to highly reproducible experimental conditions.
- Versatility: Channel flow cells can be designed for various electrode materials and geometries, making them adaptable to different research needs.
However, channel flow cells also have limitations:
- Complex Setup: The construction of channel flow cells can be intricate, requiring careful design and calibration.
- Theoretical Complexity: Analyzing the flow profiles and electrochemical behavior necessitates sophisticated theoretical models, which may not always yield exact solutions.
Wall Jet Configurations
Wall jet configurations involve a jet of electrolyte directed towards a stationary electrode, typically a disk or a flat surface. This method offers distinct advantages:
- Localized Mass Transport: The jet focuses mass transport onto a specific area of the electrode, which is beneficial for studying localized reactions.
- Simplicity: The setup is relatively straightforward compared to other hydrodynamic methods.
- Adaptability: Wall jet configurations can be modified to suit different experimental conditions and electrode materials.
Nevertheless, wall jet configurations have their drawbacks:
- Flow Non-Uniformity: The flow may not be as uniform as in RDE or channel flow cells, potentially affecting the reproducibility of the results.
- Limited Control: The flow rate and direction may be less controllable compared to channel flow cells, which can impact the experimental precision.
Rotating Disk Electrode (RDE)
The RDE remains a benchmark technique due to its ability to create a well-defined and controlled diffusion layer. Key advantages of RDE include:
- Well-Defined Diffusion Layer: The rotation of the disk electrode ensures a uniform and predictable diffusion layer, which is crucial for accurate kinetic measurements.
- Reproducibility: The rotation speed can be precisely controlled, leading to highly reproducible experimental conditions.
- Broad Applicability: RDE is suitable for a wide range of electrochemical studies, including redox reactions, catalysis, and material characterization.
However, RDE also has limitations:
- Complex Setup: The construction of RDE systems can be complex, requiring careful alignment and calibration.
- Theoretical Complexity: Analyzing the flow profiles and electrochemical behavior necessitates sophisticated theoretical models, which may not always yield exact solutions.
Suitability Based on Experimental Requirements
Choosing the most suitable electroanalytical technique depends on the specific experimental requirements and objectives. For instance:
- Rapid Reaction Studies: Channel flow cells may be preferable due to their high mass transport rates.
- Localized Reactions: Wall jet configurations offer better suitability for studying reactions in specific areas.
- Kinetic Measurements: RDE provides a well-defined diffusion layer, making it ideal for kinetic studies.
In conclusion, while RDE remains a versatile and powerful technique, researchers should consider the advantages and limitations of channel flow cells and wall jet configurations to select the most appropriate method for their specific experimental needs. Each technique offers unique benefits and challenges, and the choice should be guided by the experimental objectives and the nature of the electrochemical system under study.
Future Perspectives and Innovations in RDE Technology
The field of Rotating Disk Electrode (RDE) technology is poised for significant advancements, driven by innovations in electrode materials, rotation mechanisms, and integration with other analytical techniques. These developments are expected to enhance the capabilities of RDEs in various electrochemical research areas, including catalysis, energy storage, and environmental monitoring.
Advancements in Electrode Materials
One of the most promising areas of innovation in RDE technology is the development of new electrode materials. Traditional materials like platinum, gold, and glassy carbon have been widely used due to their stability and conductivity. However, there is a growing interest in exploring alternative materials such as graphene, metal-organic frameworks (MOFs), and nanocomposites. These materials offer improved electrochemical properties, including higher surface areas, better catalytic activity, and enhanced durability.
For instance, graphene-based electrodes have shown remarkable performance in oxygen reduction reactions (ORR) and hydrogen evolution reactions (HER). The high surface area of graphene allows for more active sites, leading to improved reaction kinetics. Similarly, MOFs, with their tunable structures and high porosity, can be designed to selectively catalyze specific reactions, making them ideal for use in RDEs.
Improved Rotation Mechanisms
The efficiency and accuracy of RDE experiments heavily depend on the rotation mechanism. Traditional RDE systems operate within a range of 400 to 10,000 rpm, but advancements in motor technology and control systems are enabling more precise and stable rotations. High-speed motors with advanced control algorithms can maintain consistent rotation speeds, even under varying experimental conditions.
Moreover, the integration of real-time monitoring and feedback systems allows for dynamic adjustments to the rotation speed, ensuring optimal experimental conditions. This level of control is particularly beneficial for studying fast kinetics and complex reaction mechanisms.
Integration with Other Analytical Techniques
The potential of RDE technology can be further harnessed by integrating it with other analytical techniques. For example, combining RDE with spectroscopic methods such as UV-Vis, Raman, and infrared spectroscopy provides insights into the chemical and structural changes occurring at the electrode surface during reactions. This multi-modal approach enhances the understanding of reaction mechanisms and the identification of intermediate species.
Additionally, the integration of RDE with mass spectrometry (MS) allows for the detection and quantification of gaseous and volatile products, expanding the scope of RDE applications to include environmental monitoring and industrial catalysis. The synergy between RDE and MS enables real-time analysis of reaction products, offering a comprehensive view of the electrochemical process.
Potential Impact on Future Research
The innovations in RDE technology are expected to have a profound impact on various areas of electrochemical research. In catalysis, the development of advanced electrode materials and improved rotation mechanisms will enable the study of novel catalysts and their reaction pathways. This will lead to the discovery of more efficient and sustainable catalysts for energy conversion and storage applications.
In environmental science, the integration of RDE with spectroscopic and mass spectrometric techniques will enhance the monitoring and analysis of pollutants and their degradation processes. This will contribute to the development of effective strategies for water and air purification.
Furthermore, the advancements in RDE technology will also benefit fundamental research in electrochemistry, providing new insights into electron transfer processes, adsorption/desorption phenomena, and multi-electron reactions. The enhanced capabilities of RDEs will facilitate the exploration of complex electrochemical systems, driving the advancement of theoretical models and experimental methodologies.
In conclusion, the future of RDE technology looks promising, with innovations in electrode materials, rotation mechanisms, and integration with other analytical techniques set to expand its applications and enhance its capabilities. These advancements will undoubtedly play a crucial role in shaping the future of electrochemical research, paving the way for new discoveries and technological breakthroughs.
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Metal Disc Electrode Electrochemical Electrode
- Electrode Polishing Material for Electrochemical Experiments
Related Articles
- Advantages of the Rotating Electrode Method
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
- Introduction to Rotating Disc Electrodes and Common Electrochemical Applications
- Understanding Electrodeposition with Electrochemical Electrodes
- Exploring Rotating Electrode Technology in Electrochemistry















