
Electrochemical Consumables
Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
Item Number : ELEG
Price varies based on specs and customizations
$12.90 / set
- Material
- High-purity graphite>99.99%
- rod material
- PTFE
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
We offer graphite disk electrodes and graphite sheet electrodes made from high-quality materials, ensuring their acid and alkali resistance. Our complete models are safe, durable, and customizable to suit your specific needs.
Technical specifications
Graphite electrode

| Features | 10*10*3, can be customized |
| Applicable temperature range | 0 ~ 60℃ |
| Rod material | PTFE |
| Material | High-purity graphite>99.99% |
Graphite Rod Electrode

| Features | 2*90, can be customized |
| Applicable temperature range | 0 ~ 60℃ |
| Rod material | PTFE |
| Material | High-purity graphite>99.99% |
Graphite disk electrode
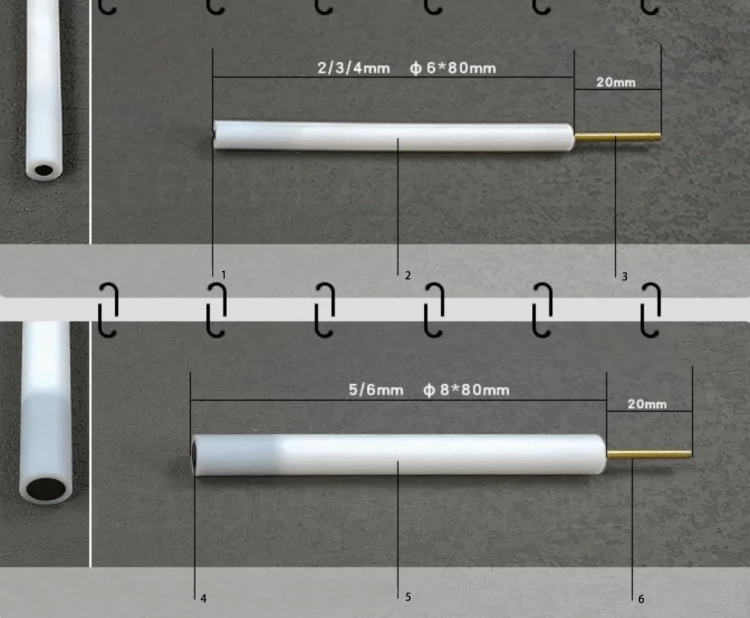
| Features | Inner core φ 2-6 |
| Applicable temperature range | 0 ~ 60℃ |
| Rod material | PTFE |
| Material | High-purity graphite>99.99% |
Detail & Parts




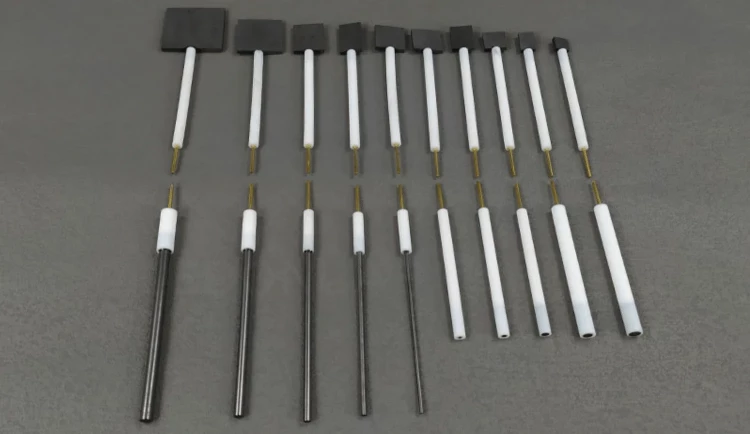
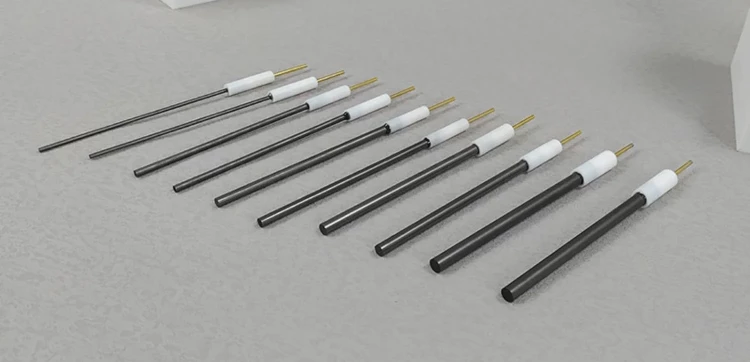

Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Is An Electrode In Electrochemistry?
What Are The Materials Used In Electrochemical Cell?
What Is The Function Of Auxiliary Electrode?
What Is Rotating Disk Electrode Used For?
What Are The 3 Electrodes In Electrochemistry?
What Are The Examples Of Electrochemical Material?
What Is The Difference Between Auxiliary And Reference Electrode?
What Is The Rotating Electrode Method?
What Are The Different Types Of Electrochemical Electrodes?
What Materials Are Commonly Used For Auxiliary Electrodes?
What Is The Rotating Ring-disk Electrode Method?
What Materials Are Commonly Used For Electrochemical Electrodes?
How Do Auxiliary Electrodes Affect The Performance Of An Electrochemical Cell?
What Are The Advantages Of Rotating Disc Electrode?
What Factors Should Be Considered When Selecting An Electrochemical Electrode?
Why Are Auxiliary Electrodes Necessary In Electrochemical Systems?
How Can Electrochemical Electrodes Be Used In Various Applications?
Are There Any Limitations Or Considerations When Using Auxiliary Electrodes?
4.7 / 5
The electrode's durability is enough to last for years in my lab. The company also offers a wide variety of models that can be customized to your specific needs.
4.8 / 5
The graphite rod electrodes I ordered were perfect for my laboratory setup. They are acid and alkali-resistant, ensuring their longevity.
4.9 / 5
KINTEK SOLUTION's graphite disk electrodes are of exceptional quality. They provide accurate and consistent results in my research experiments.
4.8 / 5
The prompt delivery of the graphite sheet electrodes was impressive. They arrived well-packaged and ready to use immediately in our laboratory.
4.7 / 5
The graphite disc electrodes are a great value for the price. They are well-made and have held up well in our lab's demanding environment.
4.9 / 5
KINTEK SOLUTION's graphite electrodes are technologically advanced and provide reliable performance in our laboratory's high-temperature experiments.
4.8 / 5
The graphite disc electrodes' high purity ensures accurate and reliable results in our laboratory's electrochemical experiments.
4.7 / 5
The graphite rod electrodes are easy to use and maintain, making them a convenient choice for our laboratory's routine experiments.
4.9 / 5
The exceptional thermal shock resistance of the graphite sheet electrodes makes them ideal for our laboratory's high-temperature applications.
4.8 / 5
The graphite disc electrodes' low specific resistance enables efficient and accurate electrochemical measurements in our laboratory.
4.7 / 5
KINTEK SOLUTION's graphite electrodes are a valuable addition to our laboratory, providing precise and reproducible results in various experiments.
4.9 / 5
The fast delivery of the graphite rod electrodes was greatly appreciated, ensuring minimal disruption to our laboratory's research schedule.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Elevate your electrochemical research with our Rotating Disk and Ring Electrodes. Corrosion resistant and customizable to your specific needs, with complete specifications.

Gold Disc Electrode
Looking for a high-quality gold disc electrode for your electrochemical experiments? Look no further than our top-of-the-line product.

Rotating Platinum Disk Electrode for Electrochemical Applications
Upgrade your electrochemical experiments with our Platinum Disc Electrode. High-quality and reliable for accurate results.

Gold Electrochemical Sheet Electrode Gold Electrode
Discover high-quality gold sheet electrodes for safe and durable electrochemical experiments. Choose from complete models or customize to meet your specific needs.

Platinum Sheet Electrode for Laboratory and Industrial Applications
Elevate your experiments with our Platinum Sheet Electrode. Crafted with quality materials, our safe and durable models can be tailored to fit your needs.

Platinum Sheet Electrode for Battery Lab Applications
Platinum sheet is composed of platinum, which is also one of the refractory metals. It is soft and can be forged, rolled and drawn into rod, wire, plate, tube and wire.

Graphite Vacuum Furnace Bottom Discharge Graphitization Furnace for Carbon Materials
Bottom-out graphitization furnace for carbon materials, ultra-high temperature furnace up to 3100°C, suitable for graphitization and sintering of carbon rods and carbon blocks. Vertical design, bottom discharging, convenient feeding and discharging, high temperature uniformity, low energy consumption, good stability, hydraulic lifting system, convenient loading and unloading.

Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Looking for a reliable quartz electrochemical cell? Our product boasts excellent corrosion resistance and complete specifications. With high-quality materials and good sealing, it's both safe and durable. Customize to meet your needs.

Vertical High Temperature Graphite Vacuum Graphitization Furnace
Vertical high temperature graphitization furnace for carbonization and graphitization of carbon materials up to 3100℃.Suitable for shaped graphitization of carbon fiber filaments and other materials sintered in a carbon environment.Applications in metallurgy, electronics, and aerospace for producing high-quality graphite products like electrodes and crucibles.

Graphite Vacuum Furnace Negative Material Graphitization Furnace
Graphitization furnace for battery production has uniform temperature and low energy consumption. Graphitization furnace for negative electrode materials: an efficient graphitization solution for battery production and advanced functions to enhance battery performance.

Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
The high thermal conductivity film graphitization furnace has uniform temperature, low energy consumption and can operate continuously.

Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
IGBT experimental graphitization furnace, a tailored solution for universities and research institutions, with high heating efficiency, user-friendliness, and precise temperature control.

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.

Flat Corrosion Electrolytic Electrochemical Cell
Discover our flat corrosion electrolytic cell for electrochemical experiments. With exceptional corrosion resistance and complete specifications, our cell guarantees optimal performance. Our high-quality materials and good sealing ensure a safe and durable product, and customization options are available.

Lab Electrochemical Workstation Potentiostat for Laboratory Use
Electrochemical workstations, also known as laboratory electrochemical analyzers, are sophisticated instruments designed for precise monitoring and control in various scientific and industrial processes.

Large Vertical Graphite Vacuum Graphitization Furnace
A large vertical high-temperature graphitization furnace is a type of industrial furnace used for the graphitization of carbon materials, such as carbon fiber and carbon black. It is a high-temperature furnace that can reach temperatures of up to 3100°C.

Carbon Graphite Boat -Laboratory Tube Furnace with Cover
Covered Carbon Graphite Boat Laboratory Tube Furnaces are specialized vessels or vessels made of graphite material designed to withstand extreme high temperatures and chemically aggressive environments.

Precision Machined Yttrium Stabilized Zirconia Ceramic Rod for Engineering Advanced Fine Ceramics
Zirconia ceramic rods are prepared by isostatic pressing, and a uniform, dense and smooth ceramic layer and transition layer are formed at high temperature and high speed.

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.
Related Articles

Electrode Materials for Rotating Ring-Disk Electrodes
Rotating ring-disk electrodes (RRDEs) are used in a wide range of applications, from fuel cells to sensors, and they require careful selection of electrode materials for optimal performance.

Electrochemical Electrodes in Chemical Analysis
Electrochemical electrodes are essential tools used in many chemical analysis techniques and experiments. These electrodes are devices that allow us to measure the electrical potential difference in a chemical reaction.

Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
Explore the detailed workings, applications, and significance of Rotating Disk Electrodes (RDE) in electrochemical research. Discover how RDEs are used in fuel cells, catalyst development, and more.

A Comprehensive Guide to Reference Electrodes
Reference electrodes are used in electrochemical measurements to establish a stable potential against which the potential of the working electrode can be measured.

Electrochemical Consumables: A Comprehensive Guide to Materials, Applications, and Selection
Discover the world of electrochemical consumables, including types of electrodes (working, auxiliary, and reference) and electrolytes, as well as factors to consider when selecting materials for your electrochemical experiments or applications.

Electrode Fixture Guide: Types, Design, and Applications
Discover the comprehensive guide to electrode fixtures, covering various types, design considerations, and their indispensable role in industries like electroplating, welding, and electrochemical cells.

The Future of Electrochemical Electrodes
The latest trends and developments in electrode materials and their implications for the future of electrochemistry.

Optimizing Performance with Graphite Vacuum Furnaces: A Comprehensive Guide
Unlock the potential of graphite vacuum furnaces for high-temperature material treatment. Learn about their efficiency, customization options, automation, and key considerations for graphite rod usage.

Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
Explore the world of reference electrodes, including calomel, silver chloride, and mercury sulfate. Understand their construction, principles, and applications in electrochemical measurements.

Understanding Electrodes and Electrochemical Cells
An electrode is a point where current enters and leaves the electrolyte. It is a conductor used to make a junction with a nonmetallic part of a circuit. Electrodes can be made of materials such as gold, platinum, carbon, graphite, or metal. They serve as the surface for oxidation-reduction reactions in electrochemical cells. There are different types of electrodes, including anode and cathode.

Introduction to Rotating Disc Electrodes and Common Electrochemical Applications
An overview of rotating disc electrodes and their applications in various electrochemical studies, including catalyst evaluation, battery research, and corrosion protection.

The Invisible Architecture of Accuracy: Mastering Electrode Installation
Master the lifecycle of electrode installation—from inspection to alignment and maintenance—to ensure safety and reproducibility in electrochemical experiments.