Hydrogen Fuel Cell Catalyst Evaluation and Research
Catalyst Development for PEM Fuel Cells
The ongoing advancements in hydrogen energy technology have significantly propelled the development of Proton Exchange Membrane (PEM) fuel cells. These fuel cells are pivotal in the electrolysis of water to produce hydrogen, a process that heavily relies on catalyst materials. Presently, the primary catalyst utilized in this domain is platinum (Pt), a precious metal known for its exceptional catalytic properties. However, the scarcity and high cost of platinum pose substantial challenges to the large-scale commercialization of hydrogen energy.
To address these limitations, extensive research is being conducted to explore alternative catalysts that reduce the reliance on platinum. This includes the development of non-platinum, non-precious metal catalysts, which are crucial for the sustainable commercialization of hydrogen energy. The significance of these efforts cannot be overstated, as they aim to overcome the resource constraints and economic barriers associated with platinum-based catalysts.
| Current Challenges | Research Focus |
|---|---|
| Scarcity of platinum | Reduction of platinum-loaded catalysts |
| High cost of platinum | Development of non-platinum, non-precious metal catalysts |
| Resource constraints | Exploration of alternative materials for large-scale commercialization |
The transition to more sustainable and cost-effective catalysts is not only a technological necessity but also a strategic imperative for the broader adoption of hydrogen energy technologies. This shift will enable the industry to meet the growing demand for clean energy solutions while mitigating the environmental and economic impacts of traditional catalyst materials.
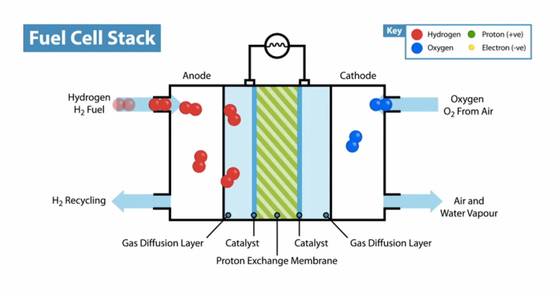
Rotating Disk Electrode Testing for Catalyst Layers
Rotating disk electrode (RDE) testing is a pivotal technique in evaluating the performance of catalyst layers in proton exchange membrane fuel cells (PEMFCs). This method allows for the precise control and measurement of mass transport phenomena, which are critical in understanding the diffusion limits and electrochemical kinetics of catalysts. The RDE operates within a three-electrode system, where the rotation of the disk electrode ensures a constant flux of reactants to the surface, enabling detailed studies of the electrode reaction mechanisms.
In the context of PEMFCs, RDE testing is particularly valuable for assessing both low- and high-loaded catalyst layers. These layers often incorporate porous micrometer carbon dry gel particles loaded with platinum (Pt) catalysts. The porosity and distribution of these particles significantly influence the diffusion of reactants and the overall efficiency of the fuel cell. By using RDE, researchers can systematically analyze how these factors impact the performance of the catalyst layers under various operational conditions.
The applications of RDE in catalyst layer research extend beyond PEMFCs. For instance, the technique is also employed in the development of non-precious metal catalysts, which aim to reduce the reliance on scarce and expensive platinum. This shift is crucial for the commercial viability of hydrogen energy technologies. Additionally, RDE experiments can be coupled with ring-disk electrode (RDE) configurations to study the homogeneous bulk reactions of intermediate species, providing deeper insights into the reaction pathways and kinetics.
In summary, rotating disk electrode testing offers a robust framework for evaluating the diffusion limits and electrochemical behavior of catalyst layers in PEMFCs. Its ability to control mass transport and provide detailed kinetic data makes it an indispensable tool in the quest for more efficient and cost-effective catalyst materials.
Lithium-Air Battery Research
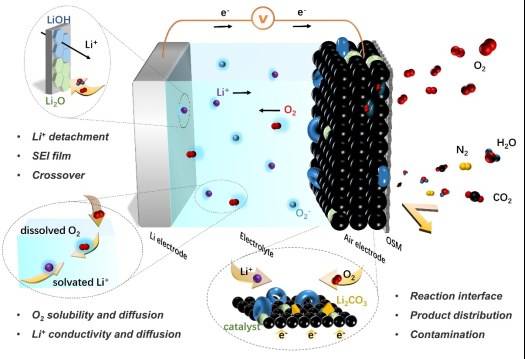
Catalyst Requirements in Lithium-Air Batteries
In lithium-air batteries, the oxygen utilized is sourced directly from the ambient environment, which means that the supply of oxygen as an anode reactant is virtually unlimited. Consequently, the capacity of these batteries is predominantly determined by the lithium electrode, specifically the cathode. To facilitate the complex electrochemical reactions within lithium-air batteries, the incorporation of a catalyst is indispensable. The efficacy of this catalyst plays a pivotal role in the overall performance of the battery.
Higher activity of the catalyst leads to enhanced charging and discharging efficiencies, as well as improved cycle life. This is because the catalyst aids in lowering the activation energy required for the reactions, thereby accelerating the rate at which these reactions occur. The more active the catalyst, the more effectively it can mediate the transfer of electrons and ions, ensuring smoother and faster electrochemical processes.
Moreover, the choice of catalyst can significantly influence the durability and longevity of lithium-air batteries. Advanced catalysts not only improve the initial performance but also help in maintaining this performance over repeated charge-discharge cycles. This dual benefit underscores the critical importance of catalyst selection in the development of high-performance lithium-air batteries.
Influence of Cathode Porosity on Oxygen Reduction
The porosity of the cathode material in lithium-air batteries significantly influences the oxygen reduction reaction (ORR), which is a critical process for the battery's performance. This influence is particularly evident when studying the ORR using a rotating circular disk electrode (RDE). The RDE allows for precise control over mass transport, enabling researchers to isolate and analyze the effects of cathode porosity on the ORR kinetics.
Key Factors Influencing ORR
-
Mass Transport Efficiency:
- High Porosity: Increases the surface area available for the ORR, facilitating faster oxygen diffusion and higher reaction rates.
- Low Porosity: Limits oxygen access to the active sites, resulting in slower reaction kinetics and reduced battery efficiency.
-
Electrochemical Surface Area (ECSA):
- Higher ECSA: Associated with increased porosity, leading to more active sites for the ORR and improved battery performance.
- Lower ECSA: Indicates a denser cathode structure, which may hinder the ORR and limit the battery's overall capacity.
-
Catalyst Utilization:
- Effective Catalyst Use: Higher porosity allows better dispersion and utilization of catalyst particles, enhancing the ORR.
- Inefficient Catalyst Use: Low porosity can lead to agglomeration of catalyst particles, reducing their effectiveness.
Experimental Observations
Using the RDE technique, researchers have observed that cathode materials with optimized porosity exhibit superior ORR performance. This is reflected in higher current densities and lower overpotentials during the ORR, indicating more efficient energy conversion.
| Porosity Level | ORR Current Density (mA/cm²) | Overpotential (mV) |
|---|---|---|
| High | 20 | 300 |
| Medium | 15 | 400 |
| Low | 10 | 500 |
These findings underscore the importance of cathode porosity in the design and optimization of lithium-air batteries, highlighting the need for careful material selection and structural engineering to enhance battery performance.
Electrochemical Kinetic Studies
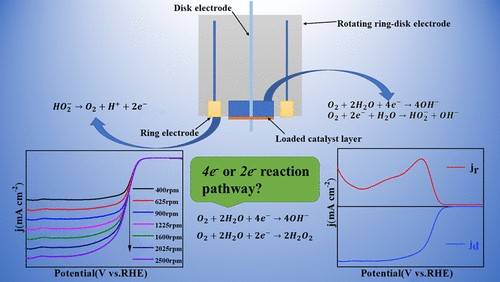
Electrode Reaction Dynamics
The focal point of electrochemical kinetics research is the intricate dynamics of electrode reactions. By precisely regulating the speed of rotating electrodes, researchers can achieve consistent mass transfer and create controlled conditions for group transfer. This meticulous control allows for the execution of detailed experimental studies on electrode reactions, yielding critical data such as polarization curves and electrochemical parameters. These empirical results are instrumental in deciphering the reaction pathways and identifying the rate-determining steps, thereby facilitating the deduction of a coherent electrode reaction mechanism.
In the realm of electrode reactions, multiple processes often occur simultaneously at the electrode surface. Researchers frequently employ limiting case analyses to simplify these complex scenarios, where the rates of specific reactions are considered negligible within a constrained potential or concentration range. Additionally, the contribution of migration to charge transport is often disregarded under certain conditions. While numerical solution techniques offer comprehensive solutions, they can be cumbersome to develop, especially for non-linear differential algebraic systems that couple electrochemical reactions with convective flow. The convergence of these numerical schemes heavily relies on judicious initial guess values, necessitating iterative solutions to the transient problem.
This approach not only enhances our understanding of the underlying reaction mechanisms but also provides a robust theoretical foundation for practical applications in industries ranging from energy production to corrosion protection.
Electrocatalytic Reaction Models
Electrocatalytic reaction models on rotating disk electrodes (RDEs) are pivotal in understanding and optimizing the performance of various electrochemical processes. These models provide a framework for analyzing the kinetics and mechanisms of reactions occurring at the electrode surface, which is crucial for the development of efficient catalysts and the design of advanced electrochemical devices.
Key Aspects of Electrocatalytic Reaction Models
-
Reaction Kinetics:
- Mass Transfer Control: The model accounts for the mass transfer limitations, where the rate of the reaction is governed by the diffusion of reactants to the electrode surface. This is particularly relevant in systems where the reactant concentration at the electrode surface is significantly lower than in the bulk solution.
- Electron Transfer Rates: The model also considers the rate of electron transfer between the electrode and the reactants, which is influenced by factors such as electrode potential, temperature, and the nature of the catalyst.
-
Catalyst Performance:
- Activity: The model evaluates the intrinsic activity of the catalyst, which is a measure of its ability to facilitate the reaction. Higher activity translates to faster reaction rates and better performance in electrochemical devices.
- Selectivity: The model can also assess the selectivity of the catalyst, determining its ability to favor one reaction pathway over others. This is critical in complex systems where multiple reaction pathways are possible.
-
Experimental Validation:
- Polarization Curves: Experimental data, such as polarization curves, are used to validate the model. These curves provide information on the current-potential relationship and help in identifying the rate-determining steps.
- Rotating Speed Effects: The model incorporates the effects of rotating speed on the reaction kinetics, allowing for the simulation of different experimental conditions and the prediction of optimal operating parameters.
Applications in Electrochemical Research
- Hydrogen Fuel Cells: The model is applied to study the electrocatalytic reduction of oxygen (ORR) in PEM fuel cells, where the efficiency of the catalyst layer is critical for overall system performance.
- Lithium-Air Batteries: In lithium-air batteries, the model helps in understanding the oxygen reduction reaction (ORR) and the influence of cathode porosity on reaction kinetics.
- Oxygen Evolution Reaction (OER): The model is used to evaluate the performance of catalysts in the OER, which is essential for water electrolysis and metal-air batteries.
By providing a comprehensive understanding of electrocatalytic reactions, these models play a crucial role in advancing the field of electrochemistry and contributing to the development of sustainable energy technologies.
Oxygen Reduction Reaction (ORR) Studies
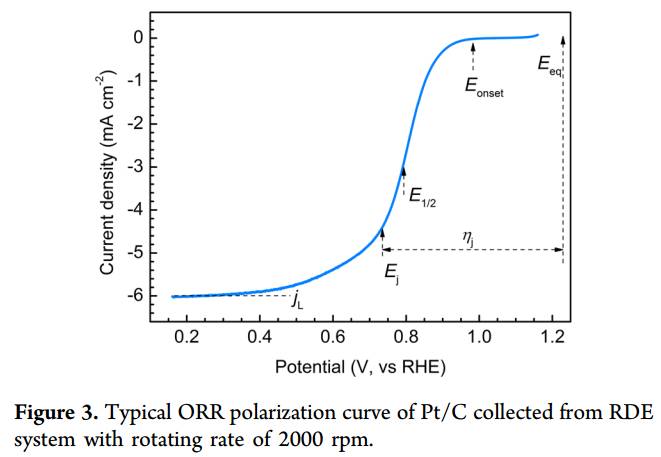
Importance of ORR in Energy Storage
The Oxygen Reduction Reaction (ORR) stands as a pivotal catalytic process within diverse energy storage technologies, such as fuel cells and metal-air batteries. This reaction is not merely a technical detail but a cornerstone of modern sustainable industrial energy storage and conversion systems. ORR's significance is multifaceted, influencing the efficiency, durability, and scalability of these technologies.
In fuel cells, ORR is critical for converting chemical energy into electrical energy. Efficient ORR catalysts can drastically reduce the overpotential, thereby enhancing the overall efficiency of the fuel cell. The development of advanced catalysts, particularly those that reduce reliance on precious metals like platinum, is a key area of research. This not only lowers costs but also addresses the scarcity of these metals, making fuel cells more viable for widespread commercial use.
For metal-air batteries, such as lithium-air batteries, ORR is equally vital. These batteries rely on oxygen from the environment, and the efficiency of the ORR directly impacts the battery's performance. High-activity catalysts can improve the charging and discharging efficiency, extending the battery's cycle life. The influence of cathode porosity on ORR further underscores the need for meticulous material selection and design in these batteries.
Moreover, the ORR's role extends beyond individual devices; it is integral to the broader landscape of sustainable energy systems. By enabling more efficient energy storage and conversion, ORR contributes to the reduction of greenhouse gas emissions and the transition towards a more sustainable energy future. The continuous advancement in ORR research and technology is thus essential for meeting the growing demand for clean and renewable energy solutions.
Measurement Techniques for ORR
The measurement of the oxygen reduction reaction (ORR) over platinum electrocatalysts using the rotating disk electrode (RDE) technique is a critical aspect of electrochemical research, particularly in the fields of fuel cells and metal-air batteries. This technique allows for the precise control of mass transport, enabling researchers to study the kinetics of the ORR under well-defined conditions.
Effect of Impurities
Impurities in the electrolyte can significantly influence the ORR performance. These impurities can act as poisons, reducing the catalytic activity of the platinum surface. For instance, trace amounts of transition metal ions or organic contaminants can adsorb onto the platinum, blocking active sites and hindering the ORR. Therefore, rigorous purification protocols are essential to ensure accurate and reproducible results.
Measurement Methodology
The RDE technique involves rotating a disk electrode at various speeds to control the diffusion layer thickness. This allows for the measurement of current as a function of potential, providing insights into the reaction kinetics. The methodology typically includes the following steps:
- Electrode Preparation: The platinum electrode is polished and cleaned to ensure a clean surface.
- Electrolyte Preparation: The electrolyte, often a solution of potassium hydroxide (KOH) or sulfuric acid (H₂SO₄), is purified to remove impurities.
- Calibration: The RDE system is calibrated using known standards to ensure accurate measurements.
- Experimental Procedure: The electrode is rotated at a constant speed, and the current-potential curves are recorded.
Applied Calibration Methods
Calibration is a crucial step to ensure the accuracy of the RDE measurements. Common calibration methods include:
- Koutecky-Levich Analysis: This method is used to separate the kinetic and diffusion-limited currents, providing a detailed understanding of the ORR mechanism.
- Butler-Volmer Equation: This equation is applied to analyze the current-potential curves, offering insights into the reaction rate constants and activation energies.
- Tafel Analysis: This technique is used to determine the Tafel slope, which provides information about the reaction mechanism and the nature of the rate-determining step.
By employing these methodologies and calibration techniques, researchers can obtain a comprehensive understanding of the ORR over platinum electrocatalysts, which is essential for the development of more efficient and durable energy storage systems.
Oxygen Evolution Reaction (OER) Studies
Role of OER in Clean Energy
The Oxygen Evolution Reaction (OER) is pivotal in the realm of clean energy, particularly in processes like water electrolysis and rechargeable metal-air batteries. These applications underscore the critical need for efficient OER catalysts to facilitate the conversion and storage of renewable energy. Despite the significant advancements, the kinetics of the OER remain sluggish, necessitating the development of superior catalyst materials to enhance reaction rates and efficiency.
Currently, the most effective OER catalysts are IrO2 and RuO2, both of which are derived from precious metals. However, the high cost and scarcity of these metals pose significant barriers to their widespread adoption. This reality underscores the urgent need to explore and develop low-cost alternatives that can match the performance and durability of existing catalysts. The pursuit of such alternatives is not merely a scientific endeavor but a strategic imperative for the broader adoption of clean energy technologies.
The development of industrially relevant, active, and durable OER catalysts is of paramount importance. These catalysts must not only reduce the reliance on precious metals but also ensure long-term stability and efficiency in various operational environments. By addressing these challenges, the field can pave the way for more sustainable and economically viable clean energy solutions.
In summary, the role of OER in clean energy is multifaceted, demanding innovative catalyst materials that can overcome the inherent limitations of current technologies. The ongoing research and development in this area are crucial for advancing the global transition to sustainable energy systems.
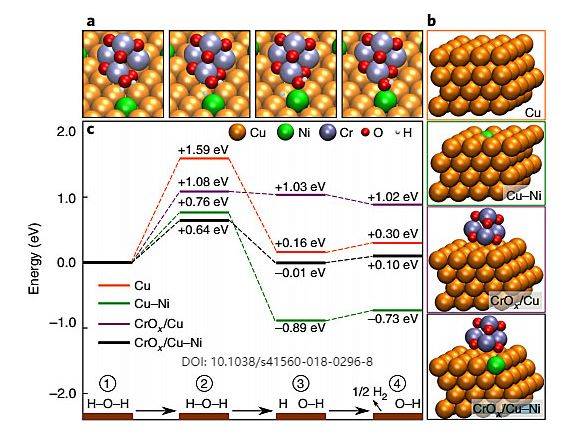
Effect of Rotating Disk Electrode on OER
The rotating disk electrode (RDE) is a pivotal tool in the study of the oxygen evolution reaction (OER) due to its ability to control the diffusion layer thickness and the flux of analyte to the electrode surface. This control is achieved by varying the rotation speed of the electrode, which directly influences the rate at which oxygen and electrolyte diffuse to the catalyst surface. For Ir nanoparticle catalysts, the RDE conditions can significantly impact the activity of the OER, making it an essential parameter in catalyst evaluation and optimization.
One of the primary functions of the RDE in OER measurements is to facilitate the rapid removal of gas bubbles formed during the reaction. This is crucial because the presence of gas bubbles can hinder the effective diffusion of reactants and the collection of accurate kinetic data. By adjusting the rotation speed, researchers can optimize the conditions to minimize bubble interference, thereby enhancing the reliability of the kinetic measurements.
Moreover, the RDE allows for the study of interfacial reaction kinetics under controlled mass transfer conditions. This capability is particularly valuable in comparing the performance of different catalyst materials, such as Ir nanoparticles, against traditional membrane and electrode assemblies. The ability to manipulate the diffusion layer thickness and analyte flux provides a nuanced understanding of the catalyst's behavior, which is essential for developing more efficient and cost-effective OER catalysts.
In summary, the RDE offers a sophisticated method for assessing the OER activity of Ir nanoparticle catalysts by controlling key experimental parameters such as rotation speed and diffusion layer thickness. This technique not only improves the accuracy of kinetic measurements but also provides insights into the catalyst's performance under various operational conditions, thereby advancing the development of next-generation OER catalysts.
Hydrogen Extraction Reaction (HER) Study
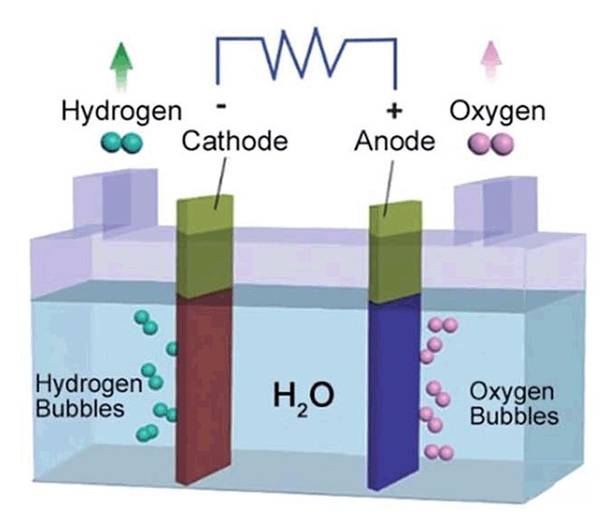
Hydrogen Production via Water Electrolysis
Hydrogen is increasingly recognized as a pivotal alternative in the quest for renewable and clean energy. Among the various methods of hydrogen production, the electrolysis of water stands out as a particularly promising avenue, often referred to as "green hydrogen." This method leverages the decomposition of water molecules into hydrogen and oxygen gases through the application of an electric current.
During the electrolysis process, protons or hydrated hydrogen ions migrate towards the cathode, where they acquire electrons, triggering a reduction reaction. This electron transfer culminates in the formation of hydrogen gas, a process scientifically termed the Hydrogen Evolution Reaction (HER). The HER is fundamental to the efficiency and sustainability of green hydrogen production, making it a focal point in contemporary hydrogen energy research.
| Component | Role in HER |
|---|---|
| Protons/Hydrated Hydrogen Ions | Migrates to the cathode, where they receive electrons. |
| Cathode | Site of electron acquisition, facilitating the reduction reaction. |
| Hydrogen Evolution Reaction (HER) | The process by which hydrogen gas is produced through reduction. |
The significance of HER extends beyond its immediate application in hydrogen production. It is intricately linked to broader energy transition strategies, aiming to reduce reliance on fossil fuels and mitigate environmental impacts. As such, advancements in catalyst development and electrode materials are crucial for optimizing the efficiency and scalability of water electrolysis, propelling the hydrogen economy forward.
Kinetic Measurements for HER
Kinetic measurements for the Hydrogen Evolution Reaction (HER) at a rotating disk electrode (RDE) are crucial for understanding the efficiency and limitations of hydrogen production via water electrolysis. The primary challenge in these measurements is the correction of the hydrogen diffusion limit, which can significantly skew the results if not properly addressed.
Hydrogen Diffusion Limit
The hydrogen diffusion limit refers to the maximum rate at which hydrogen can diffuse from the electrode surface into the bulk solution. This limit is a function of the electrode geometry, rotation speed, and the concentration gradient of hydrogen ions in the electrolyte. When this limit is reached, the reaction rate becomes diffusion-controlled, meaning that the rate of hydrogen production is no longer governed by the intrinsic catalytic activity of the electrode material but rather by the rate of mass transport.
Correction Techniques
To accurately measure the kinetic parameters of the HER, it is essential to correct for the hydrogen diffusion limit. This can be achieved through several methodologies:
-
Levich Analysis: This technique involves comparing the current density at different rotation speeds to determine the diffusion-limited current. By plotting the current density against the square root of the rotation speed, the diffusion-limited current can be extrapolated, allowing for the correction of the kinetic current.
-
Koutecký-Levich Plot: This method extends the Levich analysis by incorporating the Tafel slope, providing a more comprehensive correction for both kinetic and diffusion limitations. The plot typically involves the reciprocal of the current density versus the reciprocal of the square root of the rotation speed, enabling the separation of kinetic and diffusion contributions.
-
Transient Techniques: Using transient techniques such as chronoamperometry or cyclic voltammetry, the diffusion layer can be dynamically altered, providing insights into the diffusion-controlled regime and allowing for more precise kinetic measurements.
Practical Implications
Accurate kinetic measurements are vital for the development of efficient HER catalysts. By understanding the diffusion limitations, researchers can optimize electrode materials and geometries to enhance mass transport and improve the overall efficiency of hydrogen production. This, in turn, supports the broader goal of achieving scalable and cost-effective hydrogen energy solutions.
In summary, the correction of the hydrogen diffusion limit in kinetic measurements of the HER at an RDE is a critical step in accurately assessing the performance of catalyst materials. Through advanced analytical techniques, researchers can gain deeper insights into the reaction mechanisms and pave the way for more effective hydrogen production technologies.
Carbon Dioxide Reduction (CO2RR) Studies
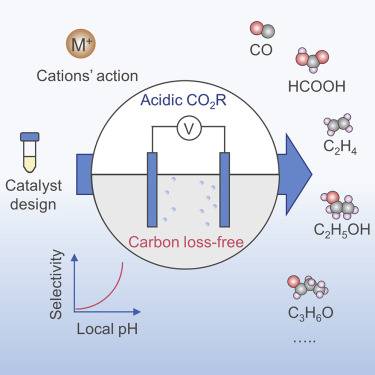
CO2 Reduction for Renewable Energy Storage
Carbon dioxide reduction (CO2RR) is a transformative technology that plays a pivotal role in converting renewable electrical energy into stored chemical bonds, thereby facilitating the production of high-value fuels and chemicals. This process is instrumental in realizing nature's 'carbon cycle' and addressing the environmental challenges posed by excessive CO2 emissions. CO2RR is not merely a reaction; it is a sophisticated mechanism that enables the synthesis of a diverse array of products, including carbon monoxide (CO), methane, formate, methanol, ethylene, and longer alkanes.
The significance of CO2RR extends beyond mere chemical synthesis. It offers a sustainable pathway to store renewable energy, which is crucial in an era where energy storage solutions are paramount. By converting CO2 into usable fuels and chemicals, CO2RR not only reduces the carbon footprint but also creates a circular economy where carbon is recycled rather than being released into the atmosphere.
Moreover, the products derived from CO2RR are versatile and can be utilized in various industrial applications. For instance, methanol and ethylene are foundational chemicals in the petrochemical industry, while longer alkanes can serve as alternatives to fossil fuels. This versatility underscores the potential of CO2RR to revolutionize the energy sector by providing a renewable and sustainable source of energy carriers.
In essence, CO2RR is more than just a scientific endeavor; it is a strategic approach to combating climate change and ensuring a sustainable future. By harnessing the power of electrochemical processes, CO2RR transforms waste CO2 into valuable resources, thereby contributing to the global effort to mitigate environmental degradation and promote sustainable development.
Electrocatalyst Development for CO2RR
In the realm of scientific inquiry, the quest for electrocatalysts that exhibit high activity, selectivity, and stability in the CO2 reduction reaction (CO2RR) is paramount. Utilizing rotating disk electrodes (RDEs) in this pursuit is not merely advantageous but essential. These electrodes facilitate precise control over mass transport and reaction kinetics, thereby enabling a more nuanced understanding of the catalytic processes involved.
The development of such electrocatalysts is a cornerstone for advancing the practical application of CO2RR technology. By leveraging RDEs, researchers can systematically evaluate the performance of various catalysts under controlled conditions, elucidating the intricate interplay between catalyst properties and reaction outcomes. This approach not only accelerates the discovery of optimal catalysts but also paves the way for scalable, efficient, and sustainable CO2 conversion processes.
Moreover, the use of RDEs in CO2RR studies allows for the identification of catalysts that can selectively produce high-value chemicals and fuels, such as carbon monoxide (CO), methane, formate, methanol, ethylene, and longer alkanes. This selectivity is crucial for maximizing the economic viability and environmental benefits of CO2 reduction technologies.
In essence, the synergy between RDEs and the development of advanced electrocatalysts for CO2RR represents a critical step towards harnessing renewable energy sources and mitigating the environmental impact of CO2 emissions.
Corrosion Inhibitor Evaluation and Research
Corrosion Inhibitor Mechanisms
Corrosion inhibitors, often referred to as "corrosion inhibitors," represent a straightforward and highly versatile strategy in the realm of metal corrosion protection. This method is extensively employed across various industries, including oil and gas extraction, machinery, chemical processing, and energy sectors. The effectiveness of corrosion inhibitors lies in their ability to mitigate corrosion by either forming a protective film on the metal surface or altering the electrochemical properties of the metal-environment interface.
One of the primary mechanisms by which corrosion inhibitors function is through the formation of a protective layer. This layer can be physical, such as a film created by the adsorption of inhibitor molecules onto the metal surface, or chemical, involving the formation of a complex compound that adheres to the metal. This protective layer acts as a barrier, preventing direct contact between the metal and the corrosive environment, thereby reducing the rate of corrosion.
Another mechanism involves the alteration of the electrochemical properties of the metal. Corrosion inhibitors can influence the electrochemical reactions at the metal surface, either by inhibiting the anodic dissolution of the metal or by reducing the cathodic reduction of oxidizing agents in the environment. This dual action can significantly reduce the overall corrosion rate, making the use of inhibitors an efficient and cost-effective solution.
The versatility of corrosion inhibitors is further highlighted by their applicability in diverse environments. Whether in acidic, alkaline, or neutral solutions, corrosion inhibitors can be tailored to suit specific conditions, ensuring robust protection for a wide range of metal substrates. This adaptability makes them indispensable in industries where metal components are exposed to varying and often harsh conditions.
In summary, the use of corrosion inhibitors is a powerful and adaptable technique in the fight against metal corrosion. By leveraging their ability to form protective layers and modify electrochemical processes, these inhibitors provide a reliable means of extending the lifespan and maintaining the integrity of metal structures across numerous industrial applications.
Rotating Cylindrical Electrode Studies
The utilization of rotating cylindrical electrodes, in conjunction with electrochemical techniques such as electrochemical AC impedance and polarization curves, offers a robust method for investigating the effects and mechanisms of corrosion inhibitors at the interface. This approach is pivotal in evaluating and screening the composition and structure of superior corrosion inhibitor materials, ultimately leading to the development of more effective corrosion inhibitor products.
By employing linear sweep voltammetry and other experiments at varying rotation rates, researchers can delve into diverse electrochemical phenomena, including multi-electron transfer processes, the kinetics of slow electron transfer, and the adsorption/desorption mechanisms of inhibitors. These studies are particularly crucial in the oil industry, where rotating cylinder experiments simulate the corrosive environment within pipelines, thereby circumventing the need for costly flow loop setups. The turbulent flow conditions generated by the rotator, even at low rotation rates, make it an ideal tool for such simulations.
Cylinders can be fabricated from a range of metals, including 1018 carbon steel, 316 stainless steel, and 430 stainless steel, to assess their performance under simulated pipeline conditions. This versatility allows for a comprehensive evaluation of different materials, providing insights into their corrosion resistance and the efficacy of various inhibitors. The ability to machine cylinder samples using actual materials further enhances the practical relevance of these studies, ensuring that the findings are directly applicable to real-world scenarios.
In summary, the integration of rotating cylindrical electrodes with advanced electrochemical methods not only deepens our understanding of corrosion inhibitor mechanisms but also facilitates the identification and development of superior corrosion inhibitor materials, thereby contributing to enhanced corrosion protection in critical industrial applications.
Corrosion Potential Studies of Metallic Materials
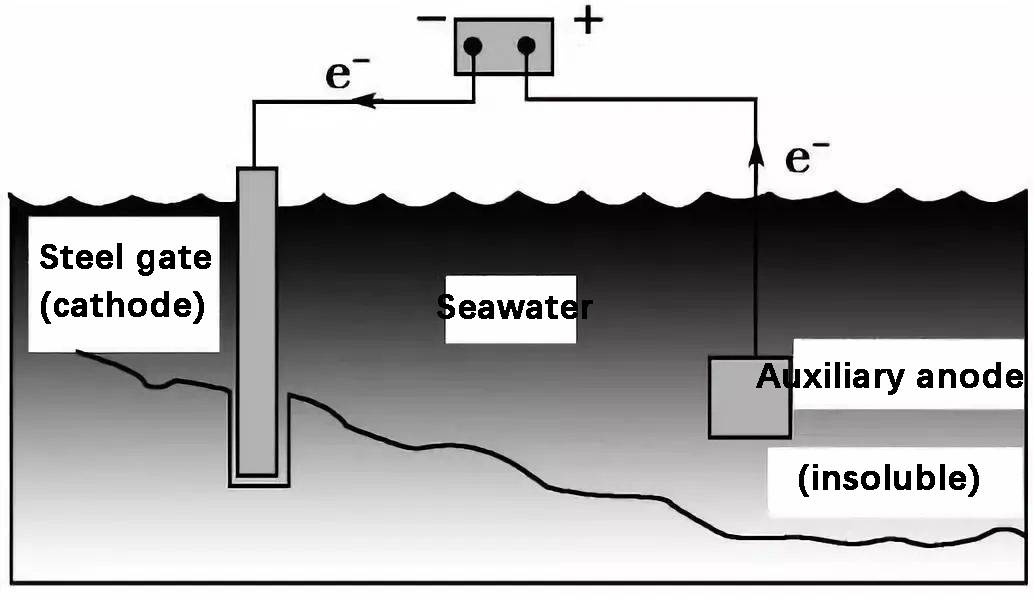
Importance of Metal Corrosion Research
Metal corrosion is a pervasive issue that affects every sector of society, leading to significant economic and safety concerns. The financial burden of corrosion is staggering, with estimates suggesting that it costs the global economy billions of dollars annually. This economic impact is not just limited to the direct costs of replacing corroded materials but also includes indirect costs such as downtime, maintenance, and loss of productivity.
In industrial settings, corrosion can escalate into severe safety hazards. For instance, corrosion in pipelines can lead to leaks, posing risks of explosions or environmental contamination. Similarly, corrosion in structural components of buildings or bridges can compromise their integrity, leading to potential collapses and endangering human lives. The safety implications are particularly critical in industries such as oil and gas, where corrosion can lead to catastrophic failures if not properly managed.
Moreover, the environmental impact of corrosion cannot be overlooked. Corroded metals often release toxic substances into the environment, contributing to pollution and harming ecosystems. This not only affects the immediate surroundings but also has long-term consequences for public health and the environment.
In summary, the study of metal corrosion is essential not only to mitigate economic losses but also to ensure safety and protect the environment. Advances in corrosion science and technology are crucial for developing effective strategies to combat corrosion, thereby safeguarding both economic interests and public well-being.
Rotating Disk Electrode Applications in Corrosion Studies
In scientific research experiments, the corrosion potential of metal materials is one of the most fundamental parameters in the study of metal corrosion and protection. This parameter provides crucial insights into the electrochemical behavior of metals when exposed to various environments, particularly in the presence of electrolyte solutions. The application of rotating disk electrodes (RDE) in these studies allows for the precise measurement of current and potential changes between the metal and the electrolyte, thereby elucidating the intricate electrochemical reactions that occur during the corrosion process.
The RDE technique is particularly advantageous in corrosion studies due to its ability to create a well-defined hydrodynamic environment. By controlling the rotation speed of the disk electrode, researchers can achieve a constant mass transfer rate, which is essential for obtaining reproducible and accurate data. This controlled environment helps in isolating the effects of mass transfer from other variables, such as convection and diffusion, thereby providing a clearer picture of the underlying electrochemical processes.
Moreover, the RDE method enables the investigation of corrosion mechanisms at the microscopic level. By analyzing the current-potential curves obtained from RDE experiments, researchers can identify the different stages of the corrosion process, including the initial activation phase, the propagation phase, and the eventual passivation of the metal surface. This detailed analysis is crucial for developing effective strategies to mitigate corrosion, such as the application of corrosion inhibitors or the use of protective coatings.
In summary, the application of rotating disk electrodes in corrosion studies offers a powerful tool for understanding the complex electrochemical interactions between metals and their environments. This knowledge is not only essential for advancing the field of corrosion science but also for developing practical solutions to protect valuable metal assets in various industrial applications.
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Disc Electrode
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
Related Articles
- Innovations in Electrochemical Electrodes Technology
- Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
- Understanding Electrodeposition with Electrochemical Electrodes
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
- Advantages of the Rotating Electrode Method

















