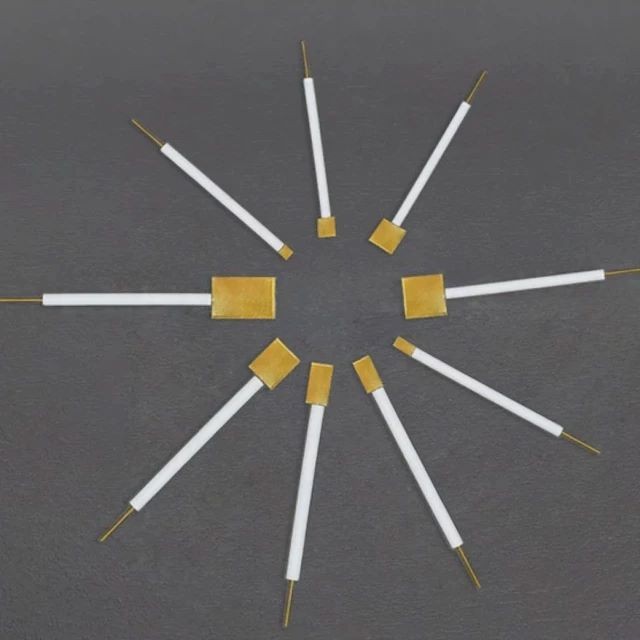
Electrochemical Consumables
Gold Electrochemical Sheet Electrode Gold Electrode
Item Number : ELEGS
Price varies based on specs and customizations
$29.90 / set
- Specifications
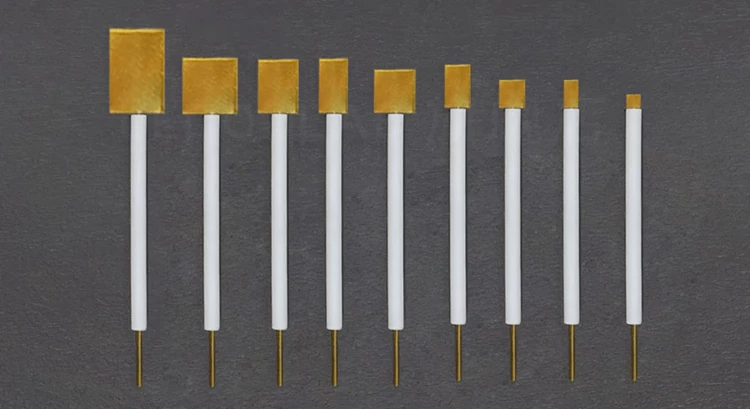
- 5*5*0.1 mm ~ can be customized
- Applicable temperature range
- 0 ~ 60℃
- Rod Material
- PTFE
- Guide material
- high purity gold> 99.99%
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
Introduction
Gold sheet electrodes are utilized in electrochemical processes, such as electroplating and electrodeposition, where a thin layer of gold is deposited onto a conductive surface. These electrodes are composed of a gold sheet bonded to a conductive substrate, typically made of materials like glass, ceramic, or metal. Gold sheet electrodes offer high electrical conductivity, corrosion resistance, and chemical inertness, making them suitable for various applications in electrochemistry, electronics, and analytical chemistry.
The gold sheet electrodes that are ideal for electrochemical experiments. Our electrodes are constructed using high-quality materials, ensuring acid and alkali resistance, as well as durability and safety. Additionally, our electrodes come in complete models and can be customized to meet your specific needs.
Technical specifications

| Specifications | 5*5*0.1 mm ~ can be customized |
| Applicable temperature range | 0 ~ 60℃ |
| Rod Material | PTFE |
| Guide material | high purity gold> 99.99% |
Detail & Parts


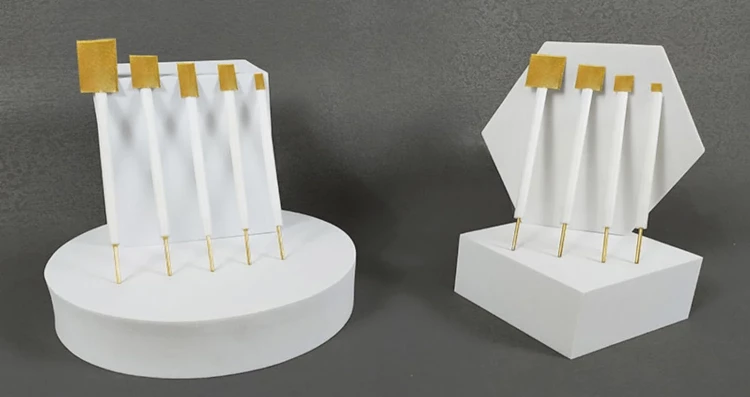
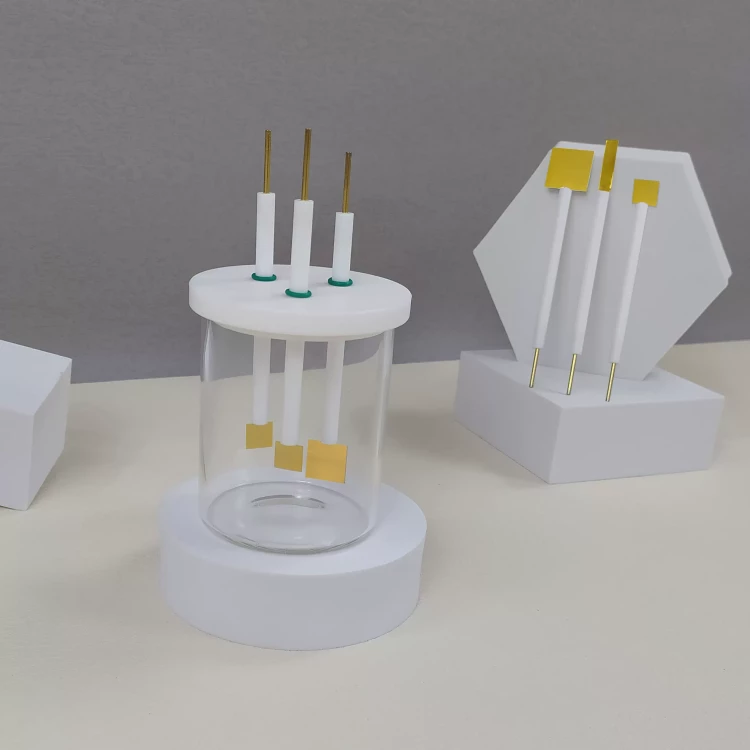
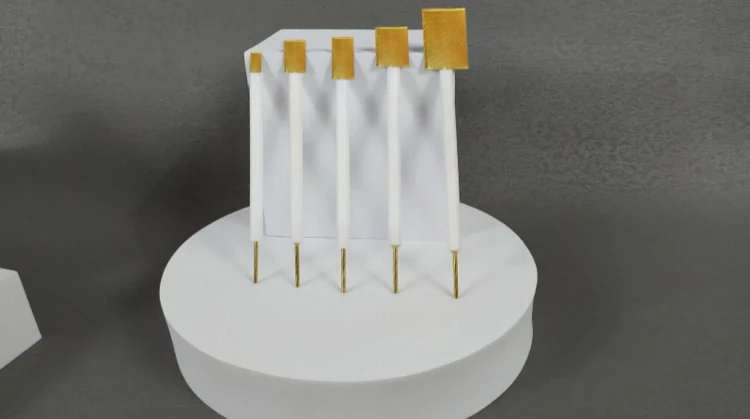
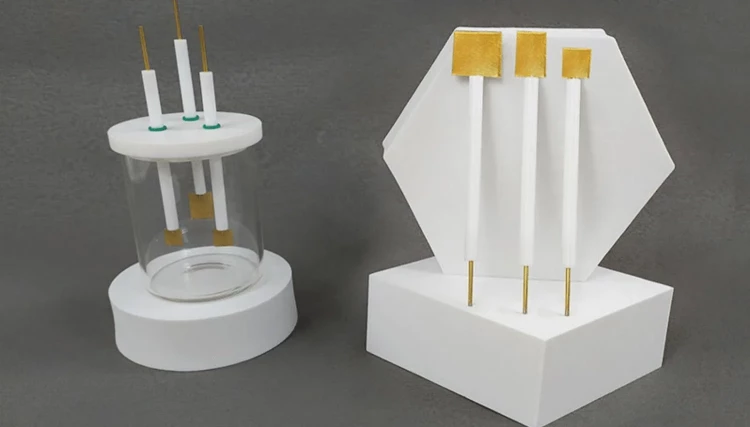
Applications
Gold sheet electrodes are commonly used in a variety of applications, including:
- Electrochemistry: Gold electrodes are inert and have a high surface area, making them ideal for electrochemical studies such as cyclic voltammetry and impedance spectroscopy.
- Electroplating: Gold is a valuable metal that can be used to coat other metals to improve their corrosion resistance, conductivity, and appearance.
- Electronics: Gold is used in electrical contacts and connectors due to its high conductivity and resistance to corrosion.
- Medicine: Gold electrodes are used in medical devices such as pacemakers and defibrillators.
- Semiconductors: Gold is used in the manufacture of semiconductors due to its high conductivity and resistance to corrosion.
Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Is An Electrode In Electrochemistry?
What Is The Function Of Auxiliary Electrode?
What Are The 3 Electrodes In Electrochemistry?
What Is The Difference Between Auxiliary And Reference Electrode?
What Are The Different Types Of Electrochemical Electrodes?
What Materials Are Commonly Used For Auxiliary Electrodes?
What Materials Are Commonly Used For Electrochemical Electrodes?
How Do Auxiliary Electrodes Affect The Performance Of An Electrochemical Cell?
What Factors Should Be Considered When Selecting An Electrochemical Electrode?
Why Are Auxiliary Electrodes Necessary In Electrochemical Systems?
How Can Electrochemical Electrodes Be Used In Various Applications?
Are There Any Limitations Or Considerations When Using Auxiliary Electrodes?
4.8 / 5
The quality of these gold sheet electrodes is top-notch! They're highly durable and resistant to acids and alkalis, making them perfect for my electrochemical experiments.
4.9 / 5
These gold sheet electrodes are incredibly reliable. I've been using them for years and they've never let me down. They're a must-have for any electrochemist.
4.7 / 5
I was impressed by the speed of delivery for these gold sheet electrodes. I ordered them one day and they arrived the next day. Amazing!
4.6 / 5
These gold sheet electrodes are a great value for the money. They're very affordable and they perform just as well as more expensive brands.
4.8 / 5
The technological advancement of these gold sheet electrodes is remarkable. They're designed to meet the demands of modern electrochemical research.
4.9 / 5
I'm very satisfied with the durability of these gold sheet electrodes. They've withstood countless experiments and they're still going strong.
4.7 / 5
These gold sheet electrodes are very easy to use. They come in complete models and can be customized to meet my specific needs.
4.6 / 5
I highly recommend these gold sheet electrodes to any electrochemist. They're a great addition to my lab.
4.8 / 5
The quality of these gold sheet electrodes is exceptional. They're made from high-purity gold and they're very durable.
4.9 / 5
These gold sheet electrodes are a great value for the money. They're very affordable and they perform just as well as more expensive brands.
4.7 / 5
I'm very impressed with the durability of these gold sheet electrodes. They've withstood countless experiments and they're still going strong.
4.6 / 5
These gold sheet electrodes are very easy to use. They come in complete models and can be customized to meet my specific needs.
4.8 / 5
I highly recommend these gold sheet electrodes to any electrochemist. They're a great addition to my lab.
4.9 / 5
The quality of these gold sheet electrodes is exceptional. They're made from high-purity gold and they're very durable.
4.7 / 5
These gold sheet electrodes are a great value for the money. They're very affordable and they perform just as well as more expensive brands.
4.6 / 5
I'm very impressed with the durability of these gold sheet electrodes. They've withstood countless experiments and they're still going strong.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Metal Disc Electrode Electrochemical Electrode
Elevate your experiments with our Metal Disk Electrode. High-quality, acid and alkali resistant, and customizable to fit your specific needs. Discover our complete models today.

Glassy Carbon Sheet RVC for Electrochemical Experiments
Discover our Glassy Carbon Sheet - RVC. Perfect for your experiments, this high-quality material will elevate your research to the next level.

Glassy Carbon Electrochemical Electrode
Upgrade your experiments with our Glassy Carbon Electrode. Safe, durable, and customizable to fit your specific needs. Discover our complete models today.

Side Window Optical Electrolytic Electrochemical Cell
Experience reliable and efficient electrochemical experiments with a side window optical electrolytic cell. Boasting corrosion resistance and complete specifications, this cell is customizable and built to last.

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.

Thin-Layer Spectral Electrolysis Electrochemical Cell
Discover the benefits of our thin-layer spectral electrolysis cell. Corrosion-resistant, complete specifications, and customizable for your needs.

Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
Experience optimal performance with our Water Bath Electrolytic Cell. Our double-layer, five-port design boasts corrosion resistance and longevity. Customizable to fit your specific needs. View specs now.

Double-Layer Water Bath Electrolytic Electrochemical Cell
Discover the temperature-controllable electrolytic cell with a double-layer water bath, corrosion resistance, and customization options. Complete specifications included.
Related Articles

Electrochemical Electrodes in Chemical Analysis
Electrochemical electrodes are essential tools used in many chemical analysis techniques and experiments. These electrodes are devices that allow us to measure the electrical potential difference in a chemical reaction.

Understanding Electrodeposition with Electrochemical Electrodes
Electrodeposition is a process of depositing a metal or a non-metallic material onto a surface by applying an electric current.

Electrolytes and Electrochemical Electrodes
Electrolytes and electrodes play an essential role in electrochemistry. Electrolytes are substances that conduct electricity when dissolved in water or melted.

Understanding Electrodes and Electrochemical Cells
An electrode is a point where current enters and leaves the electrolyte. It is a conductor used to make a junction with a nonmetallic part of a circuit. Electrodes can be made of materials such as gold, platinum, carbon, graphite, or metal. They serve as the surface for oxidation-reduction reactions in electrochemical cells. There are different types of electrodes, including anode and cathode.

Applications of Electrolytic Cells in Purification and Electroplating
Electrolytic cells are chemical cells that use electricity to generate a non-spontaneous redox reaction. These cells are used in various electrochemical processes such as electrolysis and electroplating.

Innovations in Electrochemical Electrodes Technology
Recent advancements in nanotechnology and materials science have led to significant improvements in electrochemical devices, making them more efficient, durable, and cost-effective.

The Future of Electrochemical Electrodes
The latest trends and developments in electrode materials and their implications for the future of electrochemistry.

Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
Explore the world of reference electrodes, including calomel, silver chloride, and mercury sulfate. Understand their construction, principles, and applications in electrochemical measurements.

A Comprehensive Guide to Reference Electrodes
Reference electrodes are used in electrochemical measurements to establish a stable potential against which the potential of the working electrode can be measured.

Electrode Materials for Rotating Ring-Disk Electrodes
Rotating ring-disk electrodes (RRDEs) are used in a wide range of applications, from fuel cells to sensors, and they require careful selection of electrode materials for optimal performance.

How to Choose the Right Electrochemical Electrode
The choice of electrode material can have a significant impact on the performance of the electrochemical system.

A Beginner's Guide to Understanding Reference Electrodes in Electrochemistry
Reference electrodes provide a stable and known potential that other electrodes can be compared to, allowing for accurate measurements of electrochemical reactions.