Introduction to Reference Electrodes
Reference electrodes are used in electrochemical measurements to establish a stable potential against which the potential of the working electrode can be measured. They are essential for accurate electrochemical measurements, particularly in analytical chemistry, where they are used in pH meters, ion-selective electrodes, and potentiometric titrations. Reference electrodes can be classified into aqueous and non-aqueous types, depending on the nature of the electrolyte used. The basic construction of most reference electrodes consists of a metal electrode in contact with a solution containing a known concentration of a specific ion. The selection of the appropriate reference electrode depends on the nature of the electrochemical system being studied and the specific requirements of the measurement.
Table of Contents
Types of Reference Electrodes
There are several types of reference electrodes that are used in different electrochemical applications. These reference electrodes are classified based on their composition, electrolyte, and applications. In this section, we will discuss some of the commonly used reference electrodes.

Calomel Electrode
The simplest type of reference electrode is the calomel electrode, which is composed of mercury and mercurous chloride in contact with a solution of potassium chloride. Calomel electrodes are very stable and commonly used in laboratory applications due to their stability and reproducibility. However, they contain mercury, which makes them unsuitable for use in certain applications such as food, beverage, or environmental studies.
Silver/Silver Chloride Electrode
Another commonly used reference electrode is the silver/silver chloride electrode, which consists of a silver wire coated with silver chloride in contact with a solution of potassium chloride. The silver/silver chloride electrode is widely used in laboratory applications due to its stability and reproducibility. It is also the most common type of reference system used in electrochemical measurements; however, if the sample is incompatible with silver or chloride, then a saturated calomel electrode is a suitable alternative.
Copper/Copper Sulfate Electrode
The copper/copper sulfate electrode is used in specific electrochemical measurements such as corrosion studies, pH measurements, and ion-selective electrode measurements. The copper-copper sulfate reference electrodes have three advantages over other electrodes. They have a Lexan tube, a strong top can, and a CPT ceramic plug. These CPT plugs have numerous advantages, namely uniform and controlled CPT porosity, low electrical resistance due to pre-heating on the condition that the plug is kept moist in the saturated copper-sulfate solution, and the drying and wetting process has the same low resistance.
Lead/Lead Sulfate Electrode
The lead/lead sulfate electrode is used in specific electrochemical measurements such as the determination of lead in solution. The electrode consists of a lead wire coated with lead sulfate in contact with a solution of potassium chloride.
Zinc/Zinc Sulfate Electrode
The zinc/zinc sulfate electrode is used in specific electrochemical measurements such as the measurement of zinc ion concentration. The electrode consists of a zinc wire coated with zinc sulfate in contact with a solution of potassium chloride.
In summary, a comprehensive understanding of the different types of reference electrodes and their applications is crucial for accurate and reliable electrochemical measurements. It is essential to choose the appropriate reference electrode for a specific electrochemical measurement to ensure accurate and reproducible results.
Aqueous Reference Electrodes
A reference electrode is a critical component in any electrochemical measurement system. It provides a stable and reproducible potential against which all other potentials can be measured. Aqueous reference electrodes are designed for use in aqueous solutions, and they come in several types, including the silver/silver chloride electrode, the calomel electrode, and the hydrogen electrode.
Silver/Silver Chloride Electrode
The silver/silver chloride electrode is one of the most commonly used reference electrodes in pH measurements. This electrode is made of a silver wire coated with a layer of silver chloride. The electrode is immersed in a solution of potassium chloride saturated with silver chloride. The potential of the electrode is determined by the equilibrium between the silver and silver chloride ions in the solution.
Calomel Electrode
The calomel electrode is another type of aqueous reference electrode commonly used in electroanalytical chemistry. It consists of a mercury-mercurous chloride (Hg2Cl2) electrode and a potassium chloride solution saturated with silver chloride. The potential of the electrode is determined by the equilibrium between the mercury and mercurous ions in the solution.
Hydrogen Electrode
The hydrogen electrode is the most fundamental and accurate type of reference electrode, but it requires special handling and is not practical for routine use. It consists of a platinum electrode in contact with a solution of hydrogen ions at a pressure of one atmosphere. The potential of the electrode is determined by the equilibrium between the hydrogen ions and hydrogen gas in the solution.
Considerations When Selecting an Aqueous Reference Electrode
When selecting an aqueous reference electrode, it is important to consider factors such as stability, reproducibility, and ease of use. Regular calibration and maintenance are also critical to ensure accurate and reliable measurements.
Conclusion
A comprehensive understanding of reference electrodes and their proper use is essential for any laboratory professional working with electrochemical systems. The selection of the right reference electrode type depends on the specific application requirements. Aqueous reference electrodes are available in various types, and each type has its own advantages and disadvantages. The choice of reference electrode type should be based on factors such as stability, reproducibility, and ease of use. Regular calibration and maintenance are also important to ensure accurate and reliable measurements.
Non-aqueous Reference Electrodes
Reference electrodes are an essential component of electrochemical measurements, providing a stable potential against which other electrodes can be compared. While aqueous reference electrodes are commonly used, non-aqueous reference electrodes are necessary for specific applications. These electrodes are typically composed of a metal/metal ion redox couple in a non-aqueous solvent, such as acetonitrile or dichloromethane. The choice of solvent depends on the specific redox couple and the solubility of the supporting electrolyte.

Composition of Non-Aqueous Reference Electrodes
Common non-aqueous reference electrodes include the Ag/Ag+ electrode in acetonitrile and the Fe(Cp)2+/+ electrode in dichloromethane. Non-aqueous reference electrodes are particularly useful for electrochemical measurements of non-aqueous solvents, such as in organic synthesis or battery research. However, they require careful handling to prevent contamination or degradation of the solvent or redox couple.
Challenges of Non-Aqueous Reference Electrodes
Their potential stability may be affected by changes in temperature or solvent composition. Despite these challenges, non-aqueous reference electrodes are an essential tool for researchers in electrochemistry and related fields. A comprehensive understanding of their properties and limitations is crucial for accurate and reliable electrochemical measurements.
Pseudo-Reference Electrodes
In many applications, even a small amount of electrolyte solution leaking from the reference electrode can immediately compromise the electrochemical reactions occurring in the analyte solution. Primary among these applications is non-aqueous electrochemistry. In these applications, it may be possible to use what is called a pseudo-reference electrode. The simplest pseudo-reference electrode is a metal wire, like platinum, inserted directly into the analyte solution. A reference potential will develop that is strictly due to the composition of that solution. Though this half-cell will supply a constant reference potential during a single experiment, any changes in the cell solution will result in a change in the reference potential.
Conclusion
Non-aqueous reference electrodes are an integral component of electrochemical measurements, providing a stable potential against which other electrodes can be compared. Careful handling and a comprehensive understanding of their properties and limitations are crucial for accurate and reliable electrochemical measurements. In some applications, pseudo-reference electrodes can be used to avoid issues related to electrolyte solution leakage and contamination.
Basic Construction of Reference Electrodes
Reference electrodes are vital tools in electrochemistry that are used to measure the potential difference between an electrode and the solution in which it is immersed. The basic construction of a reference electrode consists of a metal wire coated with a layer of metal chloride. The metal chloride layer acts as a source of ions that helps maintain a stable potential difference between the reference electrode and the solution being measured.
Metal Wire
The metal wire used in reference electrodes is typically made of silver or platinum. This is because these metals are inert and do not react with the solution being measured. The metal wire serves as the main electrode for the reference electrode and is responsible for conducting the electrical current.
Metal Chloride Layer
The metal chloride layer is made of various materials such as silver chloride, calomel, and saturated potassium chloride. This layer acts as a source of ions that helps maintain a stable potential difference between the reference electrode and the solution being measured. The metal chloride layer is essential in maintaining a stable potential difference because it has a known potential and is not affected by changes in the solution being measured.
Glass Body
The metal wire and metal chloride layer are enclosed in a glass body called a reference electrode body. The glass body provides mechanical support for the metal wire and metal chloride layer. It also provides insulation to prevent any electrical interference from the solution being measured.
Electrode Connection
The reference electrode is connected to the working electrode and the counter electrode, forming a complete electrochemical cell. The potential difference between the reference electrode and the solution being measured is used to calculate the potential difference between the working electrode and the solution. This enables researchers to accurately measure the electrochemical properties of solutions and materials.
Calibration and Maintenance
It is important to note that reference electrodes require careful calibration and maintenance to ensure accurate measurements. Calibration involves checking the potential difference between the reference electrode and a standard reference electrode such as the Standard Hydrogen Electrode (SHE) or the Saturated Calomel Electrode (SCE). Maintenance involves cleaning the electrode and ensuring that the metal chloride layer is intact and functioning correctly.
In summary, the basic construction of reference electrodes consists of a metal wire coated with a layer of metal chloride, enclosed in a glass body. The metal wire, metal chloride layer, and glass body work together to maintain a stable potential difference between the reference electrode and the solution being measured. Careful calibration and maintenance are essential to ensure accurate measurements.
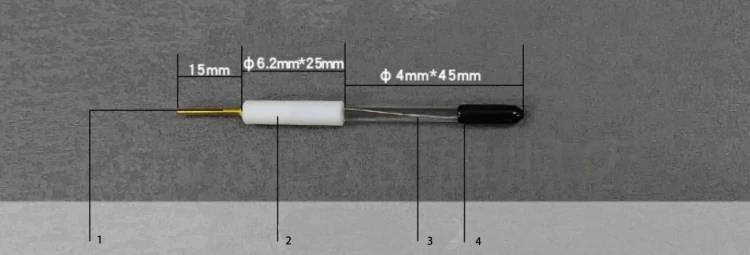
Choosing the Right Reference Electrode
Choosing the right reference electrode is crucial for obtaining accurate and reliable results in laboratory experiments. There are several factors to consider when selecting the appropriate electrode for your experiments.
Types of Reference Electrodes
Reference electrodes are classified based on their filling solutions, which can be aqueous or non-aqueous. Aqueous reference electrodes are commonly used in aqueous solutions, while non-aqueous reference electrodes are suitable for non-aqueous solutions.
Factors to Consider
Other factors to consider when choosing a reference electrode include the type of sample being analyzed, the desired accuracy and precision, and the cost of the electrode.
Compatibility with the Experimental Setup and Electrochemical System
It is also essential to consider the compatibility of the reference electrode with the experimental setup and the electrochemical system being studied.
Reference Filling Solutions
The ideal reference filling solution for any particular application should meet the following requirements:
- The filling solution's electrolyte should neither react with nor contaminate the sample.
- The filling solution should provide the dominant ions present at the liquid junction interface.
- The diffusion rates of both the cations and anions of the filling solution's electrolyte should be as close to equal as possible.
Effects of Changing the Reference Fill Solution
When the reference filling solution of a reference electrode is changed, the potential developed at the fill solution-internal reference element interface will change as well. This new potential may be less stable and/or more sensitive to temperature changes than the previous fill solution.
Crystallization
Experienced users of reference electrodes have seen many times over the formation of crystals at the bottom end of their electrodes, and know them for what they are - simply the salt crystals of the electrolyte solution used in the reference electrode. These salt crystals will not, up to a point, interfere with the electrode's performance. However, these crystals can, over periods of time, become packed together so tightly that they hinder the proper flow of reference filling solution through the junction.
Choice of Electrode Material
The choice of electrode material is critical for achieving optimal yields and selectivity in synthetic organic electrochemistry. The material imparts significant influence on the kinetics and thermodynamics of electron transfer, and frequently defines the success or failure of a transformation.
In conclusion, selecting the right reference electrode requires careful consideration of various factors. A comprehensive guide to reference electrodes can provide valuable insights to help researchers make informed decisions and obtain accurate and reliable results in their experiments.
Care and Maintenance of Reference Electrodes
Reference electrodes are critical in electrochemical measurements as they set a fixed potential for comparison with other electrodes. Here are important steps to follow to ensure the proper care and maintenance of reference electrodes:
Step 1: Proper storage
Reference electrodes should be stored in a cool and dry place to avoid contamination or damage. This prevents the electrode from being compromised by environmental factors that may affect its performance and accuracy.
Step 2: Cleaning
Before use, it is essential to clean the electrode with distilled water and dry it with a lint-free cloth. It is crucial to avoid wiping the electrode with paper towels or tissues as they can leave residues that affect the readings. Proper cleaning helps remove any dirt, grime or other contaminants that may have accumulated on the electrode.
Step 3: Calibration
Reference electrodes should be calibrated regularly to ensure that they are functioning correctly. This can be done by comparing the electrode potential readings against a known standard. If the readings are not within an acceptable range, the electrode may need to be replaced or recalibrated. Regular calibration ensures that the electrode remains accurate and reliable over time.
Step 4: Handling
Reference electrodes should be handled carefully to avoid damage to the delicate glass bulb or junction. Dropping or mishandling the electrode can cause cracks or scratches that affect its performance. It is crucial to ensure that the electrode is not subjected to any physical or mechanical stress that may alter its readings.
Step 5: Unclogging the liquid junction
If you are experiencing drifty, jumpy, or readings requiring a long stabilization time, you may be experiencing the effects of a clogged liquid junction. The first cleaning method to try is by draining the electrode of fill solution, rinsing it several times with distilled water, and refilling the electrode with its standard filling solution. If this doesn't improve the performance of the electrode, you can next try pulling a vacuum over the liquid junction to try and force the filling solution through the liquid junction. If the liquid junction remains clogged, more severe steps can be taken. The next step is to boil the liquid junction in a dilute KCl solution for 10 minutes. After 10 minutes, turn off the heat and allow the electrode to cool while immersed in the solution before the resuming of testing. The last step to try, if boiling is unsuccessful, is the physical, abrasive cleaning of the liquid junction itself. Note that this cleaning method is to be used only as a last resort, as sanding the liquid junction will severely shorten the life of the reference electrode.
In conclusion, regular care and maintenance of reference electrodes are crucial in obtaining accurate and reliable results in electrochemical measurements. Proper storage, cleaning, calibration, handling, and unclogging are essential to ensure that reference electrodes remain in good condition and deliver precise readings.
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
Related Articles
- How to Choose the Right Reference Electrode for Your Application
- A Guide to Choosing the Right Reference Electrode for Your ISE Analysis
- Electrolytes and Electrochemical Electrodes
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria











