Introduction to the Importance of Reference Electrodes
Reference electrodes are an essential component of any electrochemical measurement. They serve as a stable reference point for the potential difference between the working electrode and the solution being analyzed. Without a reference electrode, it would be impossible to accurately measure the potential of the working electrode. Reference electrodes come in various types, each with their strengths and limitations. Choosing the right reference electrode material based on factors such as cost, stability, and toxicity is crucial for obtaining reliable and accurate results. Regular testing and maintenance are also necessary to ensure the reference electrode's proper functioning.
Table of Contents
- Introduction to the Importance of Reference Electrodes
- Types of Reference Electrodes (Aqueous, Calomel, Non-Aqueous)
- Construction of Reference Electrodes
- Liquid junctions and their significance
- Choosing the Right Reference Electrode Material
- Considerations for Electrode Selection (Cost, Stability, Toxicity)
- Examples of commonly used reference electrodes
- Importance of Testing and Maintenance
- Conclusion: Selecting the Best Reference Electrode for Your Needs
Types of Reference Electrodes (Aqueous, Calomel, Non-Aqueous)
Reference electrodes are crucial in obtaining accurate and reliable results in ion-selective electrode (ISE) analysis. There are different types of reference electrodes available in the market, each with its unique properties and applications.
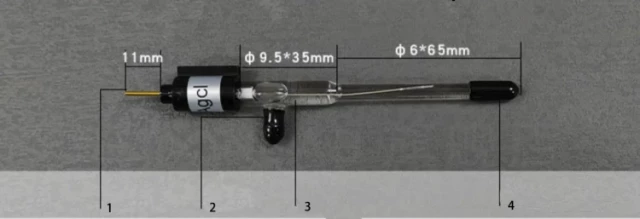
Aqueous Reference Electrodes
Aqueous reference electrodes are commonly used in aqueous solutions and are relatively inexpensive. They are made of a silver or silver chloride wire coated with a layer of silver chloride and immersed in an electrolyte solution. The most common aqueous reference electrodes used include the Standard Hydrogen Electrode, Normal Hydrogen Electrode, Saturated Calomel Electrode, Reversible Hydrogen Electrode, Silver Chloride Electrode, Copper-Copper Sulfate Electrode, PH Electrode, Dynamic Hydrogen Electrode, and Palladium-Hydrogen Electrode.
Calomel Reference Electrodes
Calomel reference electrodes are widely used in various industries due to their high stability and reproducibility. They consist of a mercury-mercurous chloride electrode immersed in a potassium chloride solution. Calomel electrodes are very stable. However, they contain mercury which makes them unsuitable for use in certain applications such as food, beverage, or environmental studies. Their disposal must also be carefully controlled due to the environmental implications.
Non-Aqueous Reference Electrodes
Non-aqueous reference electrodes, such as those used in organic solvents, are more complex, with a variety of designs and materials. They are used in situations where aqueous or calomel reference electrodes are not suitable. The most common non-aqueous reference electrode used is the quasi-reference electrode, which is made fresh for any given experiment. This reduces the problems that come with poor storage and maintenance. They are cheaper than other types of reference electrodes.
The purpose of a reference electrode is to provide a constant and defined potential. This potential is determined by the electrolyte inside the electrode and the reference element used. Using a combination pH electrode has its advantages, but it is not practical for every application. The most common reason for using a separate sensing (half-cell) and reference electrodes is if the different parts of the electrode are expected to have different lifespans.
When choosing the right reference electrode, it is important to consider the type of sample being tested, the required accuracy and precision, and the compatibility of the electrode with the analyte and solvent. Understanding the different types of reference electrodes and their properties can help you choose the right electrode for your ISE analysis, ensuring accurate and reliable results.
Construction of Reference Electrodes
Reference electrodes play a crucial role in ion-selective electrode (ISE) analysis by providing a stable and reproducible potential to which the indicating electrode potential can be compared. In order to choose the right reference electrode for a particular analysis, it is important to understand the construction of different types of reference electrodes.

Internal Element and Electrolyte-Containing Filling Solution
The typical reference electrode consists of an internal element, which is usually silver-silver chloride, surrounded by an electrolyte-containing filling solution. The filling solution is commonly potassium chloride (KCl) that has been saturated with silver chloride to prevent the silver chloride layer from becoming stripped. The filling solution is contained in either a glass or plastic body salt bridge that terminates at the liquid junction.
Importance of Keeping the Internal Element Wet
It is crucial that the internal element remains wet and surrounded by the reference electrolyte filling solution. To prevent leakage of the filling solution through the fill hole during shipment, the fill hole is sealed with either tape or a rubber grommet. This seal must be removed prior to use. If this seal is not removed, the fill solution leaks out of the liquid junction, creating a vacuum inside the electrode until the fill solution can no longer flow out of the electrode. This can result in drifting or unstable readings.
Liquid Junction
The liquid junction of a reference electrode is responsible for providing contact with the sample through its liquid junction. The liquid junction can be made of a variety of materials such as ceramic, cotton, or teflon. It is important to select the right fill solution according to the requirements of the application, so that it does not interact with the sample or cause errors in measurement such as if it acts as an interfering ion in ISE analysis.
Reference Electrode Cap
Reference electrodes are shipped with a cap containing reference fill solution covering the liquid junction. The cap is used to keep the liquid junction wet in order for the electrode to function properly.
Other Types of Reference Electrodes
Apart from the Ag/AgCl electrode and the calomel electrode, there are other reference electrodes that may be used in ISE analysis such as the saturated calomel electrode, the silver/silver sulfide electrode, and the silver/silver bromide electrode. The choice of reference electrode depends on the specific requirements of the analysis.
Importance of Proper Maintenance
It is important to properly maintain the reference electrode to ensure its performance remains optimal over time. This includes keeping the internal element wet and ensuring the electrode is properly stored when not in use.
By understanding the construction and characteristics of different types of reference electrodes, laboratory professionals can make informed decisions on selecting the right reference electrode for their ISE analysis, ensuring accurate and reliable results.
Liquid junctions and their significance

When it comes to ion-selective electrode (ISE) analysis, the reference electrode is a crucial component. It serves as the baseline against which the potential of the ion-selective electrode is measured. Choosing the right reference electrode for your ISE analysis is essential to ensure accurate and reliable results.
The role of liquid junctions
One important factor to consider is the type of liquid junction used in the reference electrode. The liquid junction is the interface between the reference electrode and the sample solution. It allows for the exchange of ions between the two solutions and helps maintain a stable potential. The type of liquid junction used can affect the stability and selectivity of the reference electrode.
Reference filling solutions
The ideal reference filling solution for any particular application should meet specific requirements. The filling solution's electrolyte should neither react with, nor contaminate the sample. The filling solution should provide the dominant ions present at the liquid junction interface. The diffusion rates of both the cations and anions of the filling solution's electrolyte should be as close to equal as possible.
Understanding ion conductivity
The ability of an ion to carry an electrical charge can be compared based on its ionic equivalent conductance (λ0, mho-cm/equivalent/liter). The choice of liquid junction type is mostly application dependent.
Types of liquid junctions
There are two basic classes of liquid junctions: Flowing junction and Diffusion junction. The first, is referred to as a "flowing" junction. This class of liquid junction allows the electrolyte in its entirety (liquid/gel and all) to make contact with the sample through the junction. Ceramic frit and salt bridge are examples of flowing junctions. The second class of liquid junctions are referred to as a "Diffusion" junction. This class of liquid junction allows only the ions of the electrolyte to pass through the junction and into the test sample. Annular ceramic, ceramic wick, and Teflon are examples of diffusion junctions.
Pros and cons of different liquid junctions
Ceramic frit junction may be more stable and resistant to clogging compared to a salt bridge junction, but it may also be less selective. Ceramic wick and Teflon liquid junctions exhibit lower flow rates and less sample contamination, but are more readily clogged. Open aperture junctions are beneficial in the monitoring of precipitation reactions, which can easily clog/foul other liquid junction styles which have a physical, porous salt bridge. Glass sleeve junctions exhibit an extremely high flow rate, offering highly stable and very low junction potentials, but require frequent refilling of the reference electrolyte, and sample contamination may be of concern for certain applications.
By carefully considering the type of liquid junction used in your reference electrode, you can ensure that your ISE analysis is accurate and reliable. The liquid junction allows the leakage of reference electrolyte filling solution into the sample, thereby completing the electrical circuit needed for potentiometric measurements. It is important to choose a liquid junction that is appropriate for your specific analysis and to properly maintain and replace the liquid junction as needed to ensure consistent results.
Choosing the Right Reference Electrode Material
Choosing the right reference electrode material is a critical step in ensuring the accuracy and reliability of your ion-selective electrode (ISE) analysis results. The reference electrode is an essential component of the ISE system, and it provides a stable reference potential against which the potential of the working electrode is measured. Here are some materials available for reference electrodes, their advantages and disadvantages, and how they can affect your analytical method.
Silver/Silver Chloride Electrodes
Silver/silver chloride electrodes are suitable for most applications and have a long lifespan. They have a stable potential and provide a reliable reference for your ISE analysis. However, they are vulnerable to contamination and can be affected by temperature changes, which can lead to measurement errors.
Calomel Electrodes
Calomel electrodes are more resistant to contamination than silver/silver chloride electrodes. They provide a stable potential and are not affected by temperature changes. However, they are not suitable for low ionic strength samples, which can cause measurement errors.
Ag/AgCl Coated Wire Electrodes
Ag/AgCl coated wire electrodes are a cost-effective alternative to silver/silver chloride electrodes. They are easy to use, and their response is stable. However, they can be prone to drift and require more frequent calibration than silver/silver chloride electrodes.
Ultimately, the choice of reference electrode material depends on the specific requirements of the analytical method. It is essential to carefully consider the advantages and disadvantages of each option before making a decision. By selecting the right reference electrode, you can ensure the accuracy and reliability of your ISE analysis results and obtain the most valuable information from your samples.
In summary, when choosing a reference electrode material, it is important to consider factors such as contamination, temperature, low ionic strength, and calibration requirements. Each material has its own advantages and disadvantages, and the choice of material depends on the specific application and the sample matrix. Therefore, it is crucial to select the right reference electrode material to ensure the accuracy and reliability of your ISE analysis results.
Considerations for Electrode Selection (Cost, Stability, Toxicity)
When performing ion-selective electrode (ISE) analysis, selecting the right reference electrode is crucial for obtaining reliable and accurate results. Here are some important considerations when selecting a reference electrode:
Cost
There are both disposable and reusable reference electrodes available on the market. While disposable electrodes may be more convenient, they can also be more expensive in the long run. Reusable electrodes, on the other hand, can be more cost-effective but require proper maintenance and storage to ensure their longevity.
Stability
Stability is another critical consideration when choosing a reference electrode. Electrodes that are stable over time will provide consistent results, while unstable electrodes can cause variability in measurements. Hence, it is essential to select a reference electrode that is stable over time.
Toxicity
It is crucial to consider the toxicity of the electrode material, especially if the analysis is being performed on biological or environmental samples. Some electrode materials, such as mercury, can be hazardous to health and the environment. Therefore, it is important to opt for materials that are not harmful to the environment and human health.
In conclusion, when selecting a reference electrode for ISE analysis, it is essential to consider the cost, stability, and toxicity of the electrode to ensure accurate and reliable results. By paying attention to these factors, one can select a suitable reference electrode that meets the requirements of the analysis.
Examples of commonly used reference electrodes
When performing ion selective electrode (ISE) analysis, it is crucial to choose the right reference electrode for accurate and reliable results. The following are examples of commonly used reference electrodes:
Silver/Silver Chloride (Ag/AgCl) Electrode
The Ag/AgCl electrode is the most widely used reference electrode and is suitable for most applications. It is composed of solid silver and its precipitated salt AgCl, which participate in the electrode reaction. The electrode is made by taking a wire of solid silver and coating it in AgCl. Then it is placed in a tube of KCl and AgCl solution. This allows ions to be formed (and the opposite) as electrons flow in and out of the electrode system. The reference electrode provides a stable electrical potential against which the sample can be compared.
Saturated Calomel Electrode (SCE)
The SCE electrode is also commonly used but is more sensitive to temperature changes and can release toxic mercury if damaged. It consists of a solid paste of Hg2Cl2 and liquid elemental mercury attached to a rod that is immersed in a saturated KCl solution. It is necessary to have the solution saturated because this allows for the activity to be fixed by the potassium chloride and the voltage to be lower and closer to the SHE. This saturated solution allows for the exchange of chlorine ions to take place. All this is usually placed inside a tube that has a porous salt bridge to allow the electrons to flow back through and complete the circuit.
Double Junction Reference Electrode
The double junction electrode is less prone to contamination but may be more expensive. It is designed to reduce the problem of contamination by placing a second solution between the reference half cell and the measurement solution. This second solution is separated from the measurement solution by a second salt bridge. The double junction design ensures that the internal filling solution does not come in contact with the sample solution directly. It is important to consider the specific requirements of the analysis, such as the type of ion being measured and the temperature of the sample, when choosing a reference electrode.
In summary, choosing the right reference electrode is crucial for accurate and reliable results in ISE analysis. The Ag/AgCl electrode is the most widely used and is suitable for most applications, while the SCE electrode is also commonly used but is more sensitive to temperature changes and can release toxic mercury if damaged. The double junction electrode is less prone to contamination but may be more expensive. By carefully selecting and using the appropriate reference electrode, researchers can ensure accurate and reliable results in their ISE analysis.
Importance of Testing and Maintenance
Regular testing and maintenance of reference electrodes are essential for accurate and reliable ISE analysis. Here are some important factors to consider:
Checking for Contamination or Damage
Contamination or damage to the reference electrode can lead to inaccurate results. It is crucial to check for any signs of contamination or damage before use. If contamination or damage is found, the electrode should be cleaned or replaced.
Calibration
Regular calibration is necessary to ensure accurate and consistent results. Calibration verifies the accuracy of the reference electrode and corrects any deviations. It is recommended to calibrate the electrode before each use and to follow the manufacturer's instructions.
Replacement
Reference electrodes have a limited lifespan and need to be replaced when necessary. Over time, the performance of the electrode may deteriorate, leading to inaccurate results. It is essential to replace the electrode according to the manufacturer's recommendations or when signs of deterioration are detected.
Compatibility
Choosing a reference electrode that is compatible with the ISE system is critical. Different ISE systems may require different types of reference electrodes. It is important to consult with the manufacturer or supplier to ensure the correct type of electrode is used.
Storage
Proper storage of the reference electrode is important. It should be stored in a clean and dry location, away from direct sunlight and heat. It should also be stored in a protective case or container to prevent damage.
Crystallization
Crystallization can occur in the reference electrode, leading to hindered flow of the filling solution through the junction. This can be remedied by draining the reference electrode of its filling solution and refilling it with distilled water. Several rinsings with distilled water may be necessary to dissolve all the crystals.
In conclusion, testing and maintenance of reference electrodes are critical for accurate and reliable ISE analysis. It is important to check for contamination or damage, calibrate the electrode regularly, replace the electrode when necessary, choose a compatible electrode, store the electrode properly, and address crystallization issues. By following these guidelines, laboratory technicians can ensure optimal performance of their reference electrodes and obtain accurate and consistent results in their ISE analysis.
Conclusion: Selecting the Best Reference Electrode for Your Needs
Choosing the right reference electrode for your ISE analysis is crucial to ensure accurate and reliable results. When making your selection, consider factors such as cost, stability, and toxicity. It's important to test and maintain your reference electrode regularly to ensure its optimal performance and longevity. Some commonly used reference electrodes include aqueous, calomel, and non-aqueous types. By understanding the construction and significance of liquid junctions, you can make an informed decision about the appropriate reference electrode material for your needs. With the right choice of reference electrode, you can ensure accurate and reliable readings in your laboratory work.
Related Products
- Copper Sulfate Reference Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Electrode Polishing Material for Electrochemical Experiments
Related Articles
- Understanding Electrodeposition with Electrochemical Electrodes
- Electrolytes and Electrochemical Electrodes
- Comprehensive Guide to Reference Electrodes: Types, Applications, and Selection Criteria
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- How to Choose the Right Reference Electrode for Your Application












