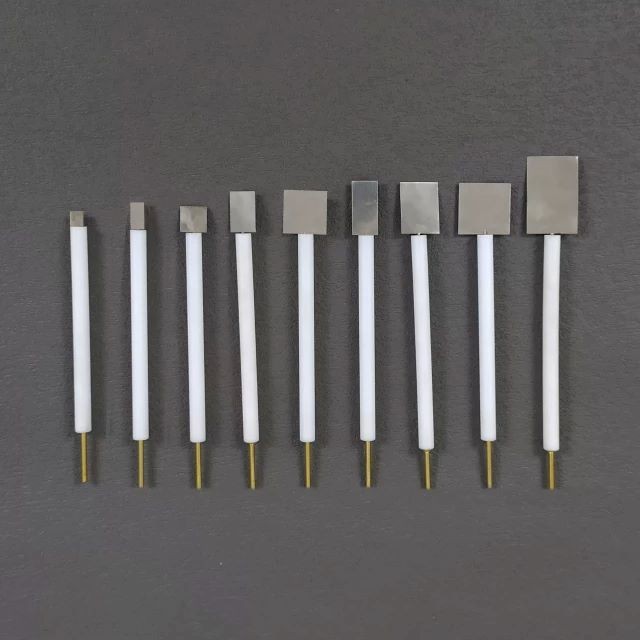
Electrochemical Consumables
Platinum Sheet Electrode for Laboratory and Industrial Applications
Item Number : ELEPS
Price varies based on specs and customizations
$21.90 / set
- Specification
- 5*5*0.1 mm, can be customized
- pplicable temperature range
- 0 ~ 60℃
- Rod Material
- PTFE
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
Introduction
A Platinum Sheet Electrode is a type of electrode that is made of a thin sheet of platinum metal. It is used in a variety of electrochemical applications, such as electroplating, electrolysis, and fuel cells. Platinum Sheet Electrodes are known for their high electrical conductivity, corrosion resistance, and catalytic activity. They are also relatively easy to fabricate and can be used in a variety of shapes and sizes.
Our platinum sheet electrodeare crafted using complete models, high-quality materials, and are both safe and durable. Additionally, they can be customized to meet your specific needs.
Technical specifications

| Specification | 5*5*0.1 mm, can be customized |
| Applicable temperature range | 0 ~ 60℃ |
| Rod Material | PTFE |
| Guide sheet material | high purity platinum> 99.99% |
Detail & Parts

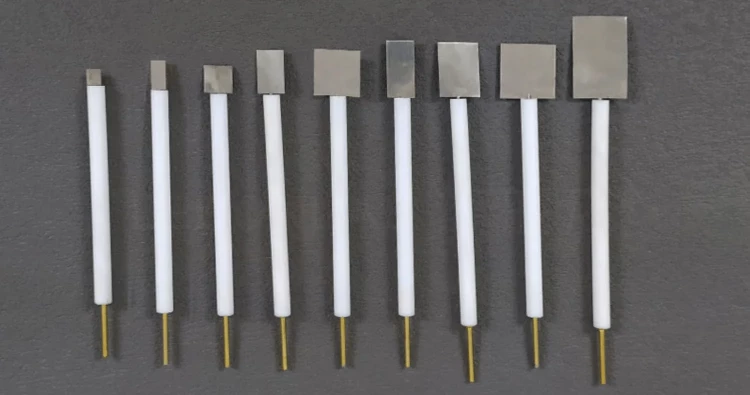
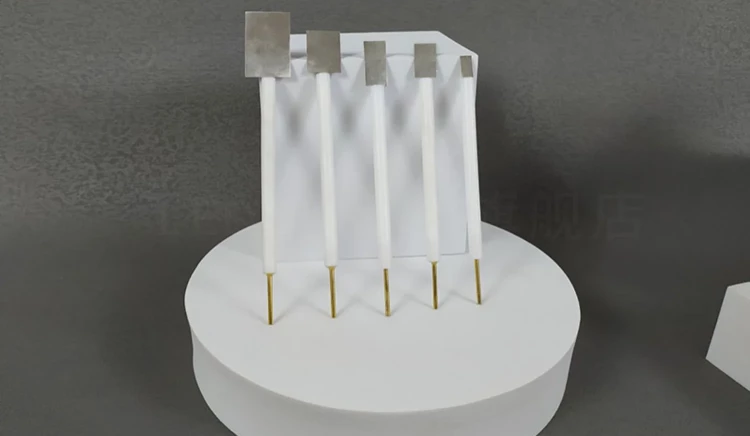


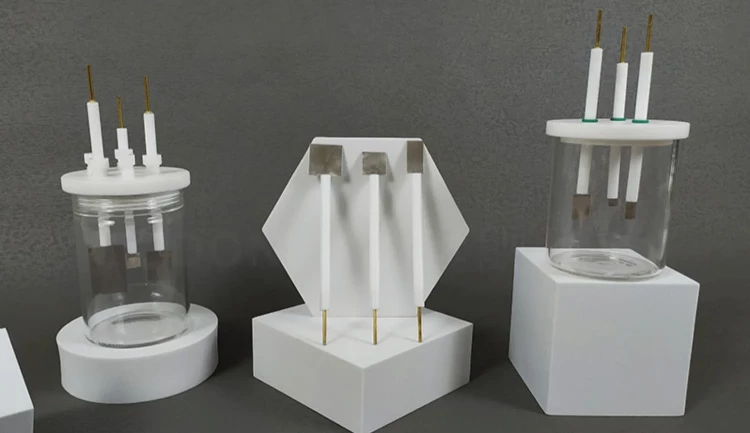
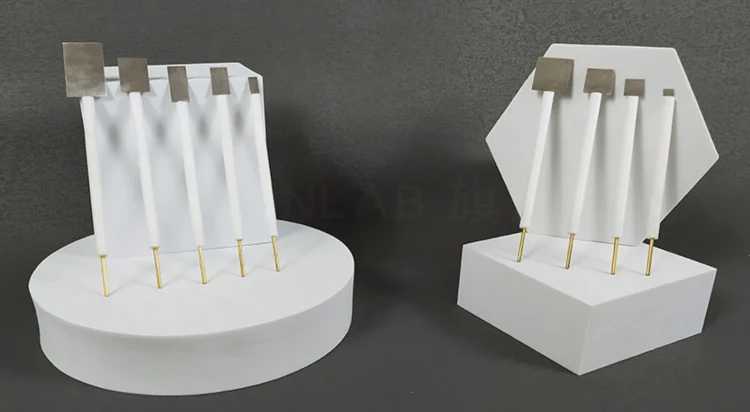


Principle
The Platinum Sheet Electrode is an inert electrode that does not react much with hydrogen. When an initial discharge allows electrons to fill into the highest occupied energy level of Pt, some of the H+ ions form H3O+ ions with the water molecules in the solution. These hydrogen and hydronium ions then get close enough to the Pt electrode (on the platinized surface of this electrode) to where a hydrogen is attracted to the electrons in the metal and forms a hydrogen atom. Then these combine with other hydrogen atoms to create H2(g). This hydrogen gas is released from the system. In order to keep the reaction going, the electrode requires a constant flow of H2(g).
Applications
Platinum sheet electrodes are widely used in a variety of applications, including:
- Fuel cells: Platinum is a key component in fuel cells, where it catalyzes the electrochemical reactions that generate electricity.
- Solar cells: Platinum is used as a counter electrode in dye-sensitized solar cells, where it helps to improve the cell's efficiency.
- Electrolysis: Platinum electrodes are used in electrolysis to produce hydrogen and oxygen from water.
- Electroplating: Platinum electrodes are used in electroplating to deposit a thin layer of platinum onto other metals.
- Chemical sensors: Platinum electrodes are used in chemical sensors to detect the presence of specific gases or ions.
- Medical devices: Platinum electrodes are used in medical devices such as pacemakers and defibrillators.
Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Is An Electrode In Electrochemistry?
What Is The Function Of Auxiliary Electrode?
What Are The 3 Electrodes In Electrochemistry?
What Is The Difference Between Auxiliary And Reference Electrode?
What Are The Different Types Of Electrochemical Electrodes?
What Materials Are Commonly Used For Auxiliary Electrodes?
What Materials Are Commonly Used For Electrochemical Electrodes?
How Do Auxiliary Electrodes Affect The Performance Of An Electrochemical Cell?
What Factors Should Be Considered When Selecting An Electrochemical Electrode?
Why Are Auxiliary Electrodes Necessary In Electrochemical Systems?
How Can Electrochemical Electrodes Be Used In Various Applications?
Are There Any Limitations Or Considerations When Using Auxiliary Electrodes?
4.8 / 5
The platinum sheet electrode arrived quickly and was packaged well. The quality of the electrode is excellent and it is performing well in our experiments.
4.9 / 5
We needed a platinum sheet electrode for our research and were impressed with the value for money of this product. It is well-made and has been a great addition to our lab.
4.7 / 5
This platinum sheet electrode is durable and has held up well to our experiments. We are very satisfied with its quality and performance.
4.8 / 5
The technological advancement of this platinum sheet electrode is impressive. It has allowed us to conduct experiments that were previously not possible.
4.9 / 5
I highly recommend this platinum sheet electrode. It is a great value for money and has exceeded our expectations in terms of quality and performance.
4.7 / 5
We have been using this platinum sheet electrode for several months now and have been very satisfied with its performance. It is a reliable and durable electrode that has met all of our needs.
4.8 / 5
The platinum sheet electrode arrived quickly and was well-packaged. We were impressed with the quality of the electrode and it has been performing well in our experiments.
4.9 / 5
This platinum sheet electrode is a great value for money. It is well-made and has been a valuable addition to our lab.
4.7 / 5
This platinum sheet electrode is durable and has held up well to our experiments. We are very satisfied with its quality and performance.
4.8 / 5
The technological advancement of this platinum sheet electrode is impressive. It has allowed us to conduct experiments that were previously not possible.
4.9 / 5
I highly recommend this platinum sheet electrode. It is a great value for money and has exceeded our expectations in terms of quality and performance.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Rotating Platinum Disk Electrode for Electrochemical Applications
Upgrade your electrochemical experiments with our Platinum Disc Electrode. High-quality and reliable for accurate results.

Platinum Sheet Electrode for Battery Lab Applications
Platinum sheet is composed of platinum, which is also one of the refractory metals. It is soft and can be forged, rolled and drawn into rod, wire, plate, tube and wire.

Platinum Auxiliary Electrode for Laboratory Use
Optimize your electrochemical experiments with our Platinum Auxiliary Electrode. Our high-quality, customizable models are safe and durable. Upgrade today!

High Purity Gold Platinum Copper Iron Metal Sheets
Elevate your experiments with our high-purity sheet metal. Gold, platinum, copper, iron, and more. Perfect for electrochemistry and other fields.

Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
High-quality graphite electrodes for electrochemical experiments. Complete models with acid and alkali resistance, safety, durability, and customization options.

Copper Sulfate Reference Electrode for Laboratory Use
Looking for a Copper Sulfate Reference Electrode? Our complete models are made of high-quality materials, ensuring durability and safety. Customization options available.

Proton Exchange Membrane for Batteries Lab Applications
Thin proton exchange membrane with low resistivity; high proton conductivity; low hydrogen permeation current density; long life; suitable for electrolyte separators in hydrogen fuel cells and electrochemical sensors.

RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Elevate your electrochemical research with our Rotating Disk and Ring Electrodes. Corrosion resistant and customizable to your specific needs, with complete specifications.
Related Articles

Applications of Electrolytic Cells in Purification and Electroplating
Electrolytic cells are chemical cells that use electricity to generate a non-spontaneous redox reaction. These cells are used in various electrochemical processes such as electrolysis and electroplating.

Understanding Electrodes and Electrochemical Cells
An electrode is a point where current enters and leaves the electrolyte. It is a conductor used to make a junction with a nonmetallic part of a circuit. Electrodes can be made of materials such as gold, platinum, carbon, graphite, or metal. They serve as the surface for oxidation-reduction reactions in electrochemical cells. There are different types of electrodes, including anode and cathode.

Electrolytes and Electrochemical Electrodes
Electrolytes and electrodes play an essential role in electrochemistry. Electrolytes are substances that conduct electricity when dissolved in water or melted.

Understanding Electrodeposition with Electrochemical Electrodes
Electrodeposition is a process of depositing a metal or a non-metallic material onto a surface by applying an electric current.

Innovations in Electrochemical Electrodes Technology
Recent advancements in nanotechnology and materials science have led to significant improvements in electrochemical devices, making them more efficient, durable, and cost-effective.

The Future of Electrochemical Electrodes
The latest trends and developments in electrode materials and their implications for the future of electrochemistry.

Electrode Materials for Rotating Ring-Disk Electrodes
Rotating ring-disk electrodes (RRDEs) are used in a wide range of applications, from fuel cells to sensors, and they require careful selection of electrode materials for optimal performance.

Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
Explore the world of reference electrodes, including calomel, silver chloride, and mercury sulfate. Understand their construction, principles, and applications in electrochemical measurements.

Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
Explore the detailed workings, applications, and benefits of quartz electrolytic cells in various industries. Learn how these cells facilitate precise chemical reactions and their role in high-purity metal production.

Understanding Electrolytic Cells: Conversion of Energy and Applications
Electrochemical cell An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or facilitating chemical reactions through the introduction of electrical energy.

A Beginner's Guide to Understanding Reference Electrodes in Electrochemistry
Reference electrodes provide a stable and known potential that other electrodes can be compared to, allowing for accurate measurements of electrochemical reactions.

Advanced Techniques in Coating Evaluation Using Electrolytic Cells
Explore the comprehensive guide on coating evaluation using electrolytic cells, covering electroplating, sol-gel methods, and wet chemical techniques. Enhance your understanding of metal coating properties and applications.