
Electrochemical Consumables
High Purity Gold Platinum Copper Iron Metal Sheets
Item Number : ELEGB
Price varies based on specs and customizations
$59.90 / set
- Specification
- customized
- Purity
- 99.99%
- Material
- customized
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
High-purity sheet metal, Gold / Platinum / copper / iron etc...Can be used in fields such as electrochemistry
Technical specifications
| Specification | customized |
| Applicable temperature range | 0 ~ 60℃ |
| Purity | 99.99% |
| Material | customized |
Detail & Parts


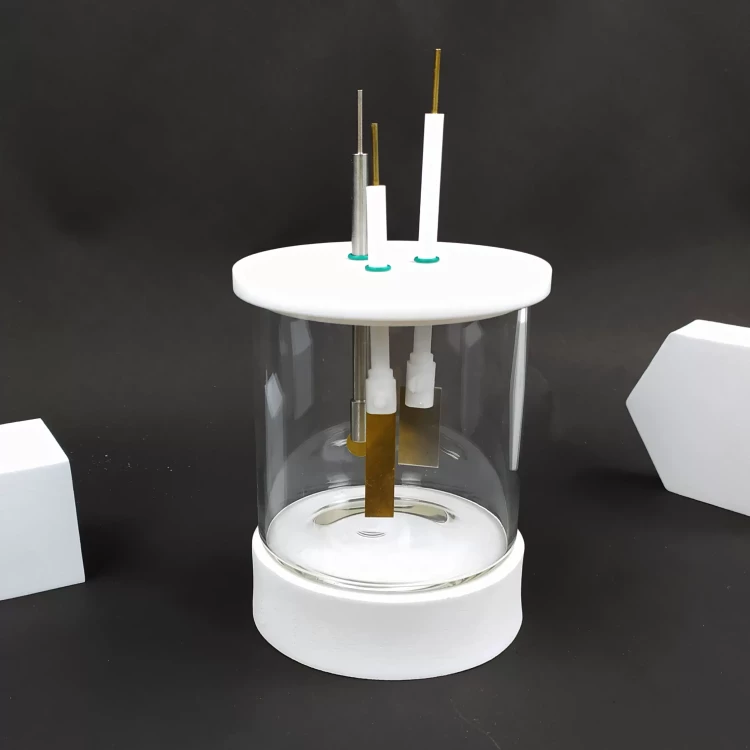

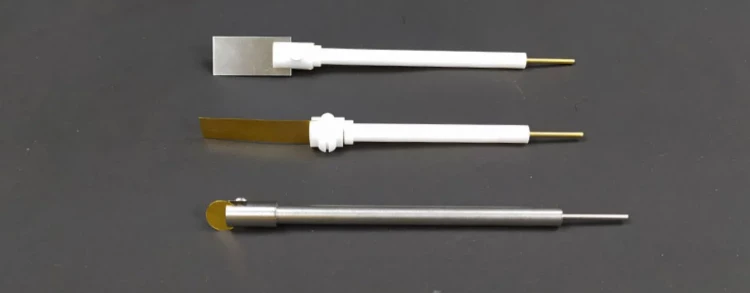

Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

4.7 / 5
KINTEK SOLUTION's high purity metal sheets are a lifesaver for our lab. They're always delivered on time and the quality is top-notch.
4.8 / 5
I've been using KINTEK SOLUTION's high purity metal sheets for years and I've never been disappointed. They're the best in the business.
4.9 / 5
The purity of KINTEK SOLUTION's high purity metal sheets is unmatched. They're perfect for our research and development projects.
4.6 / 5
I'm always impressed with the durability of KINTEK SOLUTION's high purity metal sheets. They last for years, even with heavy use.
4.7 / 5
The technological advancement of KINTEK SOLUTION's high purity metal sheets is amazing. They're always coming up with new and innovative ways to improve their products.
4.8 / 5
KINTEK SOLUTION's high purity metal sheets are the best value for money. You won't find a better deal anywhere else.
4.9 / 5
I'm so glad I switched to KINTEK SOLUTION's high purity metal sheets. They've made a huge difference in the quality of our research.
4.6 / 5
KINTEK SOLUTION's high purity metal sheets are the perfect choice for our lab. They're reliable, durable, and affordable.
4.7 / 5
I've been recommending KINTEK SOLUTION's high purity metal sheets to all my colleagues. They're simply the best.
4.8 / 5
KINTEK SOLUTION's high purity metal sheets are a game-changer for our lab. We've seen a significant improvement in our research results.
4.9 / 5
I'm so grateful for KINTEK SOLUTION's high purity metal sheets. They've made my research so much easier and more efficient.
4.6 / 5
KINTEK SOLUTION's high purity metal sheets are the gold standard. I wouldn't use anything else in my lab.
4.7 / 5
I'm a huge fan of KINTEK SOLUTION's high purity metal sheets. They're the perfect combination of quality, durability, and affordability.
4.8 / 5
KINTEK SOLUTION's high purity metal sheets are the best I've ever used. They're worth every penny.
4.9 / 5
I'm so glad I made the switch to KINTEK SOLUTION's high purity metal sheets. They've made a world of difference in my research.
4.6 / 5
KINTEK SOLUTION's high purity metal sheets are the best in the business. I highly recommend them to anyone looking for high-quality metal sheets.
4.7 / 5
I'm so impressed with KINTEK SOLUTION's high purity metal sheets. They're the perfect choice for my lab.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Platinum Sheet Electrode for Battery Lab Applications
Platinum sheet is composed of platinum, which is also one of the refractory metals. It is soft and can be forged, rolled and drawn into rod, wire, plate, tube and wire.

Professional Cutting Tools for Carbon Paper Cloth Diaphragm Copper Aluminum Foil and More
Professional tools for cutting lithium sheets, carbon paper, carbon cloth, separators, copper foil, aluminum foil, etc., with round and square shapes and different sizes of blades.

High Purity Alumina Granulated Powder for Engineering Advanced Fine Ceramics
Ordinary alumina granulated powder is alumina particles prepared by traditional processes, with a wide range of applications and good market adaptability. This material is known for its high purity, excellent thermal stability and chemical stability, and is suitable for a variety of high-temperature and conventional applications.
Related Articles

Applications of Electrolytic Cells in Purification and Electroplating
Electrolytic cells are chemical cells that use electricity to generate a non-spontaneous redox reaction. These cells are used in various electrochemical processes such as electrolysis and electroplating.

Advanced Electrolytic Cell Techniques for Cutting-Edge Lab Research
Electrolytic cells are devices that utilize an electric current to induce a non-spontaneous chemical reaction.

Unlocking Purity: The Ultimate Guide to Handheld Precious Metal Analyzers
Discover the power of XRF990 handheld precious metal analyzer in accurately testing gold, silver, platinum purity. Ideal for jewelers, recyclers, and quality inspectors.

Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
Explore the world of reference electrodes, including calomel, silver chloride, and mercury sulfate. Understand their construction, principles, and applications in electrochemical measurements.

Electrochemical Consumables: A Comprehensive Guide to Materials, Applications, and Selection
Discover the world of electrochemical consumables, including types of electrodes (working, auxiliary, and reference) and electrolytes, as well as factors to consider when selecting materials for your electrochemical experiments or applications.

Applications of H-Type Electrolytic Cell in Metal Extraction
H-type electrolytic cells uses an electrolyte solution to dissolve the metal ions and an electric current to separate the metal ions from the solution.

How to Choose the Right Electrochemical Electrode
The choice of electrode material can have a significant impact on the performance of the electrochemical system.

Electrochemical Electrodes in Chemical Analysis
Electrochemical electrodes are essential tools used in many chemical analysis techniques and experiments. These electrodes are devices that allow us to measure the electrical potential difference in a chemical reaction.

Understanding the Features and Functions of Laboratory Press
Laboratory presses are essential equipment in various industries, offering precise and controlled sample preparation for testing and research purposes. These presses come with a range of features and functions that ensure reliable and consistent results. Understanding the capabilities of laboratory presses is crucial for businesses looking to optimize their sample preparation processes. From uniform temperature distribution to mechanical solidity, these presses offer a comprehensive solution for consistent sample thickness and closure force.

Understanding Electrolytic Cells and Their Role in Copper Purification and Electroplating
Electrolytic cells play a crucial role in various industrial processes, including copper purification and electroplating. These cells utilize an external power source to drive chemical reactions, resulting in the decomposition of substances. Through the process of electrolysis, an electric current is passed through a liquid or solution containing ions, causing them to break down.

Electrode Fixture Guide: Types, Design, and Applications
Discover the comprehensive guide to electrode fixtures, covering various types, design considerations, and their indispensable role in industries like electroplating, welding, and electrochemical cells.

How To Turn XRF analysis sample preparation Into Success
In X-ray fluorescence (XRF) analysis, sample preparation is an important step because it can significantly impact both the quality and the efficiency of the analysis.