Introduction
Table of Contents
- Introduction
- Definition of Electrolytic Cell
- Concept of Electrolysis
- Role of Direct Current Power Source in Electrolytic Cells
- Definition of Electrolyte
- Roles of Anode and Cathode in Electrolytic Cells
- Movement of Ions in Electrolytic Cells
- Application of Electrolysis in Copper Production
- Process of Copper Purification via Electrolysis
- Final Results of Copper Purification Process
- Conclusion
Electrolytic cells play a crucial role in various industrial processes, including copper purification and electroplating. These cells utilize an external power source to drive chemical reactions, resulting in the decomposition of substances. Through the process of electrolysis, an electric current is passed through a liquid or solution containing ions, causing them to break down. In this blog post, we will explore the concept of electrolytic cells, the movement of ions within them, and their application in copper production. So, let's dive in and understand the fascinating world of electrolytic cells!
Definition of Electrolytic Cell
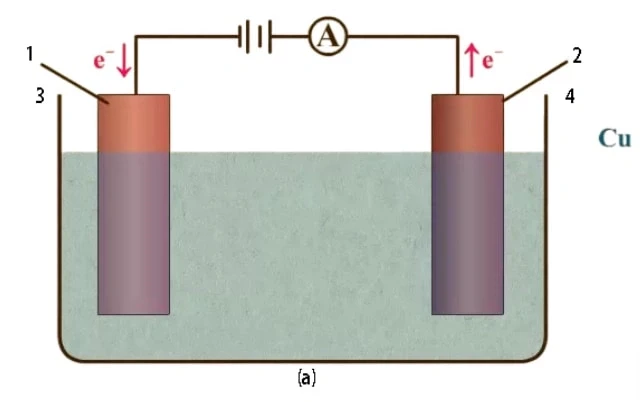
An electrolytic cell is an electrochemical cell that uses an external power source to drive a non-spontaneous redox reaction. It is commonly used in processes such as electrolysis, where chemical compounds are decomposed. The electrolytic cell consists of two electrodes, an anode (positively charged electrode) and a cathode (negatively charged electrode), immersed in an electrolyte solution. The external source of electrical energy, such as a battery, is applied between the electrodes to force the chemical reaction to occur. This is different from a galvanic cell, which is a source of electrical energy itself.
Electrolysis and its Applications
Electrolysis is a technique that utilizes an electrolytic cell and a direct electric current to drive chemical reactions that would not occur spontaneously. One important application of electrolysis is the decomposition of water into hydrogen and oxygen. This process is used in the production of hydrogen gas, which can be used as a clean and renewable energy source. Another significant application is the electrolysis of bauxite to obtain aluminum and other chemicals.
Electroplating is another common application of electrolysis. It involves the deposition of a layer of metal onto a surface using an electrolytic cell. Copper, silver, nickel, and chromium are some of the metals that can be electroplated onto various objects.
Electrochemical Cells and Redox Reactions
An electrochemical cell is a device that generates electricity from a spontaneous oxidation-reduction reaction or uses electricity to drive a non-spontaneous reaction. These cells operate based on the principles of electrochemistry, which studies the relationship between electricity and chemical reactions.
In a redox reaction, electrons are transferred from one species to another. If the reaction is spontaneous, it releases energy, which can be harnessed to do useful work. In contrast, a non-spontaneous reaction requires an input of energy to occur. Electrochemical cells facilitate these reactions by either generating electrical energy from the chemical reactions or using electrical energy to drive the reactions.
Components of Electrolytic Cells
The primary components of an electrolytic cell are the cathode, anode, and electrolyte. The cathode is the negatively charged electrode, while the anode is the positively charged electrode. The electrolyte serves as the medium for the exchange of electrons between the cathode and the anode.
Commonly used electrolytes in electrolytic cells include water containing dissolved ions and molten sodium chloride. The flow of electrons in the electrolytic cell overcomes the activation energy barrier of the non-spontaneous redox reaction, allowing the desired chemical transformation to take place.
Conclusion
In summary, an electrolytic cell is an electrochemical device that uses electrical energy to drive a non-spontaneous redox reaction. It is commonly used in processes such as electrolysis and electroplating. Understanding the principles of electrochemistry and the components of electrolytic cells is essential in utilizing these cells for various applications.
Concept of Electrolysis
Electrolysis is the process of passing an electric current through a liquid or solution containing ions, leading to the decomposition of substances.
Product of Electrolysis
Electrolysis involves the use of electricity to break down or decompose a compound, usually an ionic compound in either the molten state or an aqueous solution. The products of electrolysis can be atoms, molecules, ions, or gases. The type of electrode and electrolyte used determines the specific product of the electrolysis process.
In electrolysis, electrodes play a crucial role in conducting electricity to or from the cell through the movement of electrons. There are two types of electrodes:
-
Anode: The positive electrode is called the anode. Negatively charged ions move towards the anode, and oxidation takes place at the anode.
-
Cathode: The negative electrode is called the cathode. Positively charged ions move towards the cathode, and reduction takes place at the cathode.
Electrodes in Electrolysis
During electrolysis, an electric current is passed into and out of an electrolyte solution to restore the flow of ions necessary for a non-spontaneous reaction. The process involves immersing and separating electrodes at a distance. The electric current, supplied by a power source, travels through the electrolyte, completing the electrical circuit.
A direct current (DC) is used to drive the electrolysis reaction. This current causes ions in the electrolyte to be attracted towards the oppositely charged electrode, the cathode, and anode. The amount of electrical energy required is equal to the change in Gibbs free energy of the reaction, along with any losses in the system.
Process of Electrolysis
The process of electrolysis involves the use of an electrolytic cell, which consists of positive and negative electrodes held apart and immersed in a solution containing positively and negatively charged ions. The substance to be transformed can form the electrode, constitute the solution, or be dissolved in the solution.
The negatively charged electrode, known as the cathode, serves as the entry point for the electric current. Components of the solution migrate to the cathode, combine with electrons, and undergo reduction, resulting in the formation of neutral elements or new molecules.
Conversely, components of the solution also move towards the positively charged electrode, called the anode. At the anode, these components release their electrons and undergo oxidation, leading to the formation of neutral elements or new molecules.
If the substance to be transformed is the electrode itself, the reaction often involves the dissolution of the electrode as it gives up electrons.
Electrolysis is a fundamental process in chemistry that enables the decomposition and transformation of substances through the application of electric current. It has played a significant role in the discovery and understanding of various elements in modern chemistry.
Role of Direct Current Power Source in Electrolytic Cells
Electrolytic Cells
To understand the role of a direct current power source in electrolytic cells, we first need to grasp the concept of electrolysis. Electrolysis is a process in which an electric current is passed through a liquid containing ions, causing the compounds within the liquid to break down. This method is commonly used to separate metals from metallic compounds, isolate other chemical substances, electroplate metals, and recharge batteries.
For electrolysis to occur, a complete circuit is necessary to continuously draw electricity from the cell. An electrolytic cell consists of two stable electrodes, the cathode and the anode, which are connected to a power source. These electrodes, along with a fluid electrolyte solution, are essential components of every electrolytic cell. The electrolyte solution conducts electricity because the dissolved ions can move freely within the solution.

Definition: Electrolysis
Electrolysis is a process in which an electric current is passed through a liquid or a solution containing ions, causing the substances within to decompose.
To sustain an electrolytic reaction, a complete circuit is required to continuously obtain power from a battery or power supply. This necessitates the ability of ions to move freely within the electrolyte.
In electrolytic cells, a direct current power source is used, meaning that the electrodes are always either positive or negative. This polarity is crucial for the electrolytic process to occur effectively.
An electrolyte refers to a substance or mixture that conducts electricity and can undergo electrolysis.
When a direct current is passed through the electrolyte in an electrolytic cell, electrolysis takes place. In the case of copper purification, the impure copper anode is oxidized and forms Cu2+ ions. At the cathode electrode, the positive copper ions undergo reduction, resulting in the production of pure copper metal. Impurities in the form of other metals do not dissolve but instead form a solid sludge at the bottom of the container.
Types of Electrochemical Cells
Electrochemical cells can be classified into three types: rechargeable cells, non-rechargeable cells, and fuel cells.
Fuel cells require an external supply of fuel, which serves as the source of chemical energy needed to generate electricity. On the other hand, rechargeable and non-rechargeable batteries have the fuel stored internally within the battery itself.
Understanding the role of a direct current power source in electrolytic cells is crucial for harnessing the power of electrolysis in various industrial processes, such as metal purification and electroplating. By utilizing a direct current power source, electrolytic cells enable the decomposition of substances and the creation of pure elemental substances or other chemicals.
Definition of Electrolyte
An electrolyte is a type of substance or mixture that contains mobile ions that can undergo electrolysis. This means that when an electric current is passed through an electrolyte, the substance within it undergoes a chemical change. Electrolytes can exist in the form of a salt solution or a molten salt.

Anode and Cathode
In the context of electrolysis, the terms anode and cathode refer to the electrodes involved in the process. The anode is the electrode from which electrons flow, while the cathode is the electrode to which electrons flow. In an electrolytic cell, the anode is the positive electrode.
Conductivity of Electrolytes
Electrolytes are able to conduct electricity because they contain ions that are free to move. When an electric current is applied, anions in the electrolyte move towards the anode and are oxidized, while cations move towards the cathode and are reduced. This movement of ions allows for the flow of electrical charge through the electrolyte.
Types of Electrolytes
There are two main types of electrolytes: strong electrolytes and weak electrolytes. Strong electrolytes completely dissociate into ions when dissolved in a solution, while weak electrolytes only partially dissociate into ions.
Examples of strong electrolytes include soluble salts, acids, and bases. These substances allow electrical current to flow between the anode and cathode in a battery or electrolytic cell. On the other hand, weak electrolytes do not fully dissociate into ions in a solution.

Electrochemical Cell
An electrochemical cell is a device that converts electrical energy into chemical energy and vice versa. It consists of an electrolytic solution, salt bridge, and electrodes. The electrolyte in a battery is the substance that allows the flow of electrical current between the anode and cathode. Electrolytes can be in the form of fluids or solids.
In an electrochemical cell, current flows through the electrolyte in the form of ions rather than electrons. Negatively charged ions, called anions, flow from the cathode to the anode, while positively charged ions, called cations, flow from the anode to the cathode. This two-way flow of ions enables the transfer of electrical charge through the electrolyte.
Electrolysis
Electrolysis is the process in which an electric current is passed through a substance to induce a chemical change. It is commonly performed in an electrolytic cell, which consists of positive and negative electrodes immersed in a solution containing positively and negatively charged ions.
During electrolysis, electrons enter through the cathode, causing components of the solution to combine with the electrons and undergo reduction. At the same time, components of the solution travel to the anode, give up their electrons, and undergo oxidation. The products of these reactions can be neutral elements or new molecules.
In some cases, the substance to be transformed may form the electrode, constitute the solution, or be dissolved in the solution. Regardless of the specific setup, electrolysis allows for the controlled manipulation of chemical reactions through the application of an electric current.
Roles of Anode and Cathode in Electrolytic Cells

Anode: Electrode that provides electrons to the external circuit
The anode in an electrolytic cell plays a crucial role in the electrochemical process. It is the electrode where oxidation takes place, meaning electrons flow out from the anode into the external circuit. This flow of electrons allows for the transfer of charge and the completion of the electrical circuit within the cell.
In electrolytic cells, the anode is positively charged. It serves as a source of electrons that are needed for the reduction reactions to occur at the cathode. The anode is typically made of a reactive material, such as copper, silver, or gold, that can disassociate in the electrolyte and facilitate the flow of ions.
Cathode: Electrode that accepts electrons from the external circuit
On the other hand, the cathode is the electrode that accepts electrons from the external circuit in an electrolytic cell. It is the negative electrode where reduction reactions take place. The electrons flowing into the cathode from the external circuit combine with the oxidizing agent present in the cell, leading to the reduction of the oxidizing agent.
The cathode is essential for the overall functioning of the electrolytic cell. It serves as the site where the desired chemical reactions occur, resulting in the desired products. In the case of electrolysis, the cathode is negatively charged to attract positively charged cations towards it. Once the cations reach the cathode, they can be reduced by gaining electrons, leading to the formation of the desired products.
Both the anode and cathode are crucial components of electrolytic cells and are found in various electrical devices such as batteries, fuel cells, photovoltaic cells, electrolytic cells, and diodes. The electrolyte, which serves as a conduit for electron flow between the cathode and anode, plays a significant role in facilitating the electrochemical reactions.
Understanding the roles of the anode and cathode in electrolytic cells is essential for comprehending the working principles of these devices. The anode facilitates oxidation reactions, while the cathode enables reduction reactions, leading to the desired chemical transformations. By harnessing the power of electrolysis, various applications, such as the production of pure copper, can be achieved efficiently and effectively.
Movement of Ions in Electrolytic Cells
Cations: Travel towards the cathode and are reduced
The cathode is the positive electrode in an electrolytic cell. It is where the reduction reaction takes place. Cations, which are positively charged ions, travel towards the cathode and are reduced. This means that they gain electrons at the cathode. This reduction reaction is essential in various industrial processes, such as the production of high purity copper and other metals like sodium, magnesium, and aluminum. In these processes, cations are electrolyzed to produce the desired metal.
Anions: Travel towards the anode and are oxidized
The anode is the negative electrode in an electrolytic cell. It is where the oxidation reaction occurs. Anions, which are negatively charged ions, travel towards the anode and are oxidized. This means that they lose electrons at the anode. The oxidation of anions can lead to the liberation of gases like oxygen or the deposition of certain elements. For example, in the electrolysis of copper and silver salts, the cations are reduced at the cathode, while in the electrolysis of potassium, sodium, and calcium salts, hydrogen gas is liberated due to the reduction of water.

In summary, in electrolytic cells, cations travel towards the cathode and are reduced, while anions travel towards the anode and are oxidized. This movement of ions is crucial for the various electrochemical processes that occur in electrolytic cells.
Cathode
The cathode, the positive electrode in an electrolytic cell, plays a crucial role in the reduction reaction that takes place. It is the site where electrons flow from the electrical circuit into the non-metallic part of the cell. At the cathode, cations are reduced as they absorb the electrons coming from the wire connected to the cathode. This reduction reaction is important in processes like the production of high purity copper and other metals.
Electrolysis
Electrolysis is the process that occurs in an electrolytic cell, where electrical energy is used to perform non-spontaneous chemical reactions. In electrolysis, oxidation occurs at the anode, which is the positive plate, while reduction occurs at the cathode, which is the negative plate. The electrolytic cell converts electrical energy into chemical energy through these redox reactions.
Conduction within an electrolytic cell
In an electrolytic cell, current passes through the external circuit as electrons move, but within the cell itself, a different process occurs. Positive ions from the electrolyte gain electrons at the cathode and undergo reduction, while negative ions migrate to the positive electrode (anode) where they release electrons and undergo oxidation. This movement of ions and electrons within the cell is how the electrolysis process takes place.
Types of Electrolytes
Electrolytes can be classified as strong or weak, depending on the extent to which they ionize in an aqueous solution. Strong electrolytes ionize to a great extent and conduct a strong electric current, while weak electrolytes ionize to a small extent and conduct a small amount of electric current.
Anode
The anode is the electrode through which the conventional current enters into an electrochemical cell. It is the site where oxidation reactions occur. Negative ions or anions are prone to react and give off electrons at the anode due to their electrical potential. The electrons then move through the circuit. In a galvanic cell, the anode is negative, and the electrons largely move towards the outer part of the circuit.
Examples of electrodes
There are two types of electrodes: reactive electrodes and inert electrodes. Reactive electrodes participate in the reaction occurring in the cell and can disassociate in the electrolyte. Examples of reactive electrodes include copper, silver, and gold.
In conclusion, understanding the movement of ions in electrolytic cells is crucial in comprehending the electrochemical processes that occur in these cells. Cations travel towards the cathode and are reduced, while anions travel towards the anode and are oxidized. This movement of ions and the accompanying electron flow is the basis of electrolysis, where electrical energy is used to drive non-spontaneous chemical reactions.
Application of Electrolysis in Copper Production
Extraction of Metals
Electrolysis is widely used in the extraction of metals. There are two methods of extracting metals using electrolysis:

-
Extraction of Zinc: In this process, the zinc ore is treated with sulphuric acid to obtain a zinc sulphate solution. The solution is then electrolyzed in a tank, with aluminum cathodes and lead anodes. The current density is maintained at 1000 A/m2, and zinc is deposited on the cathode. The energy consumption for this process is approximately 3000 to 5000 KWH per tonne.
-
Extraction of Aluminium: The ores of aluminium, such as bauxite and cryolite, are chemically treated and reduced to aluminium oxide. The aluminium oxide is then dissolved in fused cryolite and electrolyzed in a furnace. Aluminium deposits at the cathode and settles down at the bottom. The temperature of the furnace is maintained at around 1000°C, and a current of about 4000 amperes is required. The energy consumed for this process is approximately 20,000 to 25,000 KWH per tonne.
Refining of Metals
Electrolysis is also used for the refining of metals. The main advantages of using electrolytic processes for refining metals are the high purity of the obtained product (around 98 to 99%) and the ability to further refine the metal. In the refining process, the anode is made of the extracted metal, and pure metal is deposited at the cathode. The electrolyte used is made of the metal solution, such as copper sulphate for copper refining and nickel chloride for nickel refining. The energy consumption for copper refining by electrolytic process is approximately 150 to 300 KWH per tonne of refined copper.
Overall, electrolysis plays a crucial role in the extraction and refining of metals, ensuring high purity and quality of the final product.
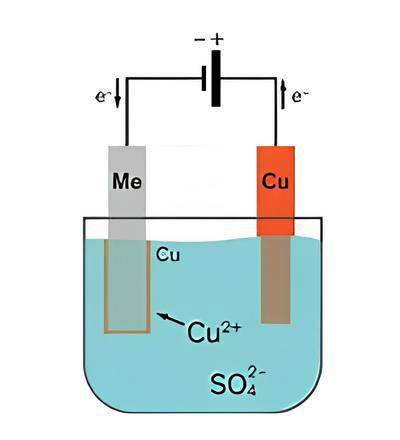
Process of Copper Purification via Electrolysis
Impure copper plates are placed in a bath of copper(II) sulfate solution
In the process of copper purification via electrolysis, impure copper plates are first produced from copper ore extraction. However, these impure copper plates are not pure enough for use in electronics and other critical applications. To obtain purer copper plates, electrolysis is used.
The impure copper plates are placed in a bath of copper(II) sulfate solution. This solution acts as the electrolyte in the electrolysis process. The impure copper plates are connected to the positive terminal of an external power supply, while a pure copper plate is connected to the negative terminal of the power supply.
External power supply drives reactions leading to plating of the cathode with copper
The external power supply drives two reactions in the electrolysis process. At the anode (the impure copper plate), copper is oxidized and dissolved into the electrolyte as Cu2+ ions:
Cu(s) ⇒ Cu2+(aq) + 2e–
This oxidation reaction causes the impure copper anode to lose mass as copper dissolves into the electrolyte.
At the cathode (the pure copper plate), copper ions in the electrolyte are reduced by gaining electrons to form copper metal:
Cu2+ (aq) + 2e– ⇒ Cu(s)
This reduction reaction causes copper metal to be plated onto the cathode, resulting in the cathode gaining mass.
The impurities present in the impure copper plates are left behind in the electrolyte. Soluble impurities remain in the electrolyte, while insoluble impurities either stay in the anode or fall to the bottom of the reaction vessel.
The process of electrolysis is widely used in the production of pure copper. Copper is a highly conductive metal used in various applications such as circuits, wires, pipes, and cooling units. It is also known for its malleability and ductility.
Electrolysis plays a crucial role in the final purification step of copper production. By using an electrolytic cell with a copper(II) sulfate solution as the electrolyte, impure copper plates can be transformed into high purity copper plates. This process ensures that the copper plates meet the required purity standards for their intended applications.
Overall, electrolysis is an important method in metallurgy, allowing the conversion of impure copper into high purity copper. It is also used for the extraction and refining of other metals such as sodium, magnesium, and aluminum. Electrolysis is a versatile process that enables the production of pure metals on a large scale.
Final Results of Copper Purification Process
Impure copper anode loses mass

During the copper purification process, the impure copper anode undergoes oxidation and loses mass. Copper atoms from the anode dissolve into the electrolyte solution as Cu2+ ions.
Pure copper cathode gains mass
At the same time, pure copper is plated onto the cathode. Copper ions from the electrolyte solution are reduced at the cathode, resulting in the deposition of pure copper metal. As a result, the pure copper cathode gains mass throughout the purification process.
Soluble impurities remain in electrolyte
During the purification process, soluble impurities present in the impure copper anode do not deposit onto the cathode. Instead, they remain in the electrolyte solution. This allows for the separation of impurities from the purified copper.
Insoluble impurities stay in anode or fall to bottom of reaction vessel
Insoluble impurities, such as other metals or solid particles, either stay embedded in the impure copper anode or fall to the bottom of the reaction vessel. These impurities do not dissolve into the electrolyte solution and are physically separated from the purified copper.
The purification process involves the use of electrolysis, where electrical energy is used to drive the chemical reactions. The impure copper anode acts as the source of copper ions, while the pure copper cathode serves as the site for copper deposition. By carefully controlling the process, the impurities are left behind, resulting in high-purity copper.
It is important to note that the electrolyte used in the purification process, such as copper sulfate, plays a crucial role in facilitating the movement of ions and enabling the separation of impurities. Additionally, the temperature and current used during electrolysis affect the efficiency of the purification process.
Overall, the final results of the copper purification process include the loss of mass from the impure copper anode, the gain of mass by the pure copper cathode, the retention of soluble impurities in the electrolyte, and the separation of insoluble impurities either in the anode or at the bottom of the reaction vessel. These results lead to the production of high-purity copper, making electrolytic purification an essential method in the metallurgical industry.
Conclusion
In conclusion, electrolytic cells play a crucial role in copper purification and electroplating. By using an external power source to drive reactions, electrolysis allows for the decomposition of substances in a liquid or solution containing ions. The anode provides electrons to the external circuit, while the cathode accepts electrons. Cations travel towards the cathode and are reduced, while anions travel towards the anode and are oxidized. In the process of copper purification, impure copper plates are immersed in a copper sulfate solution, and the cathode is plated with pure copper. The result is a reduction in impurities in the anode and the production of high-quality copper.
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Side Window Optical Electrolytic Electrochemical Cell
- Optical Water Bath Electrolytic Electrochemical Cell
- Flat Corrosion Electrolytic Electrochemical Cell
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Unseen Variable: Mastering the Electrolytic Cell Inspection
- Electrochemistry The Science Behind Electrochemical Cells
- Applications of H-Type Electrolytic Cell in Metal Extraction
- Applications of Electrolytic Cells in Purification and Electroplating