Introduction to Electrochemical Consumables
Table of Contents
Electrochemical consumables play a crucial role in electrochemistry experiments and applications. They include electrodes, which facilitate the transfer of electrons, and electrolytes, which provide ions for the electrochemical reactions. This comprehensive guide explores the world of electrochemical consumables, encompassing different types of electrodes (working, auxiliary, and reference) and electrolytes. It delves into factors to consider when selecting materials for electrochemical experiments or applications, ensuring successful and accurate results.
Types of Electrodes
Working Electrode
The working electrode (WE) is the electrode at which the electrochemical reaction of interest takes place. It is typically made of a noble metal, such as gold, platinum, or carbon, which are resistant to corrosion and have high electrical conductivity. The choice of material for the WE depends on the specific application. For example, gold is often used for electrochemical sensing applications, while platinum is commonly used for fuel cell applications.
Auxiliary Electrode
The auxiliary electrode (AE) is used to complete the electrical circuit in an electrochemical cell. It provides a pathway for current to flow between the WE and the reference electrode. The AE is typically made of a metal that is not easily oxidized or reduced, such as platinum, carbon, copper, or stainless steel. The choice of material for the AE is not as critical as that of the WE, but it should be compatible with the electrolyte and the WE material.
Reference Electrode
The reference electrode (RE) is used to provide a stable and reproducible reference potential against which the potential of the WE can be measured. The RE is typically made of a metal that is easily oxidized or reduced, such as silver, calomel, or mercury. The choice of material for the RE depends on the specific application. For example, silver/silver chloride (Ag/AgCl) REs are commonly used in aqueous solutions, while calomel REs are often used in non-aqueous solutions.

Electrolytes and Their Properties
Electrolytes are substances that contain mobile ions when molten or in aqueous solution, enabling them to conduct electricity. They play a crucial role in electrochemical processes, such as in batteries, fuel cells, and electroplating.
Types of Electrolytes
Electrolytes can be classified into three main types based on their physical state:
-
Liquid Electrolytes: These are the most common type of electrolytes and are typically solutions of salts, acids, or bases in a solvent such as water. Examples include sodium chloride (NaCl) dissolved in water, sulfuric acid (H2SO4) dissolved in water, and potassium hydroxide (KOH) dissolved in water.
-
Solid Electrolytes: Solid electrolytes are typically ionic compounds that conduct electricity in the solid state. They are often used in solid-state batteries and fuel cells. Examples include lithium-ion conducting ceramics and polymer electrolytes.
-
Molten Electrolytes: Molten electrolytes are salts that are melted at high temperatures, allowing them to conduct electricity. They are used in high-temperature applications, such as molten salt reactors and certain types of batteries. Examples include molten sodium chloride (NaCl) and molten lithium chloride (LiCl).
Factors to Consider When Selecting an Electrolyte
When selecting an electrolyte for a particular application, several factors must be considered:
-
Conductivity: The conductivity of an electrolyte is a measure of its ability to conduct electricity. It is influenced by the concentration of ions in the electrolyte and the mobility of those ions.
-
Stability: The electrolyte should be stable under the operating conditions of the electrochemical cell. It should not decompose or react with the electrodes or other components of the cell.
-
Compatibility with Electrodes: The electrolyte should be compatible with the electrodes used in the electrochemical cell. It should not corrode or dissolve the electrodes.
-
Cost: The cost of the electrolyte is also an important consideration, especially for large-scale applications.
Applications of Electrolytes
Electrolytes have a wide range of applications, including:
-
Batteries: Electrolytes are used in batteries to provide a medium for the transport of ions between the positive and negative electrodes.
-
Fuel Cells: Electrolytes are used in fuel cells to facilitate the electrochemical reactions that generate electricity.
-
Electroplating: Electrolytes are used in electroplating to deposit a thin layer of metal on a surface.
-
Chemical Processing: Electrolytes are used in various chemical processes, such as the production of chlorine and sodium hydroxide.

Considerations for Material Selection
Material selection for electrodes and electrolytes in electrochemical systems is a critical step that significantly impacts the performance, efficiency, and cost-effectiveness of the overall process. Several factors must be considered when choosing appropriate materials, including their inherent properties, reactivity, inertness, corrosion resistance, and cost.
Desirable Properties for Anode, Cathode, and Electrolyte Materials
Anode Materials:
- Efficient Reducing Agent: The anode material should possess strong reducing capabilities to facilitate the desired electrochemical reactions.
- High Coulombic Output: It should exhibit high coulombic efficiency, ensuring efficient utilization of the active material and minimizing capacity loss.
- Good Conductivity: High electrical conductivity is essential for efficient charge transfer and minimizes energy losses due to ohmic resistance.
- Stability: The anode material should be chemically and electrochemically stable under the operating conditions to prevent degradation and maintain long-term performance.
- Ease of Fabrication: The material should be easily processed and fabricated into desired shapes and dimensions, enabling cost-effective manufacturing.
- Low Cost: An economically viable anode material is crucial for large-scale applications, especially in industrial settings.
Commonly used anode materials include metals such as zinc, lithium, and graphite, selected based on their specific properties and suitability for the intended application.
Cathode Materials:
- Efficient Oxidizing Agent: The cathode material should possess strong oxidizing capabilities to facilitate the desired electrochemical reactions.
- Stability in Electrolyte: It should be chemically and electrochemically stable when in contact with the electrolyte to prevent degradation and maintain long-term performance.
- Useful Working Voltage: The cathode material should exhibit a suitable working voltage range that aligns with the desired operating conditions and ensures efficient energy storage or conversion.
- Ease of Fabrication: Similar to anode materials, the cathode material should be easily processed and fabricated into desired shapes and dimensions for cost-effective manufacturing.
- Low Cost: Economic viability is a key consideration for cathode materials, especially in large-scale applications.
Commonly used cathode materials include metallic oxides, such as lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC), and lithium iron phosphate (LFP), chosen based on their specific properties and suitability for the intended application.
Electrolyte Materials:
- Ionic Conductivity: The electrolyte should exhibit high ionic conductivity to facilitate efficient ion transport and minimize resistance to charge flow.
- Chemical and Electrochemical Stability: The electrolyte should be chemically and electrochemically stable under the operating conditions to prevent decomposition and maintain long-term performance.
- Wide Electrochemical Window: The electrolyte should possess a wide electrochemical window, allowing for a broad range of operating voltages without undergoing decomposition or other undesirable reactions.
- Compatibility with Electrodes: The electrolyte should be compatible with the anode and cathode materials, ensuring stable interfaces and preventing unwanted reactions.
- Non-Toxic and Environmentally Friendly: The electrolyte should be non-toxic and environmentally friendly, minimizing potential hazards and facilitating safe handling and disposal.
Commonly used electrolytes include aqueous solutions, organic solvents, ionic liquids, and solid-state electrolytes, selected based on their specific properties and suitability for the intended application.
Factors Influencing Material Selection
In addition to the desirable properties mentioned above, several other factors influence the selection of materials for electrodes and electrolytes:
- Reactivity: The reactivity of the materials should be carefully considered to ensure compatibility with the intended electrochemical reactions and prevent unwanted side reactions.
- Inertness: The materials should be inert towards the other components of the electrochemical system, such as the electrolyte and current collectors, to minimize corrosion and maintain long-term stability.
- Corrosion Resistance: The materials should exhibit good corrosion resistance under the operating conditions to prevent degradation and ensure reliable performance over time.
- Cost: The cost of the materials is a significant factor, especially for large-scale applications, and must be balanced against the desired performance and long-term cost-effectiveness.
By carefully considering these factors and selecting appropriate materials, researchers and engineers can optimize the performance, efficiency, and cost-effectiveness of electrochemical systems for various applications.
Applications of Electrochemical Consumables
Electrochemical consumables are specialized materials and components utilized in various industries for conducting electrochemical experiments and analyses. They play a crucial role in electrochemical processes, enabling the study of chemical reactions and the behavior of substances in solutions.
Energy Storage
Electrochemical consumables find extensive applications in energy storage systems, including batteries, fuel cells, and supercapacitors. These consumables are essential components of these devices, providing the necessary electrochemical reactions for energy storage and release.
-
Batteries: Electrochemical consumables are used in batteries to facilitate the electrochemical reactions that generate electricity. Common battery types include lead-acid batteries, lithium-ion batteries, and nickel-cadmium batteries. These consumables include electrodes, separators, and electrolytes.
-
Fuel Cells: Fuel cells utilize electrochemical reactions to generate electricity from fuels such as hydrogen or methanol. Electrochemical consumables in fuel cells include electrodes, electrolytes, and catalysts.
-
Supercapacitors: Supercapacitors store electrical energy through electrostatic attraction. Electrochemical consumables in supercapacitors include electrodes and electrolytes.
Electroplating
Electroplating is a process that uses electrochemical reactions to coat a metal surface with a thin layer of another metal. This coating can provide protection against corrosion, enhance electrical conductivity, or improve the appearance of the metal. Electrochemical consumables used in electroplating include:
-
Anodes: Anodes are the positively charged electrodes in the electroplating process. They are typically made of the metal that is being deposited onto the surface.
-
Cathodes: Cathodes are the negatively charged electrodes in the electroplating process. They are typically made of the metal that is being coated.
-
Electrolytes: Electrolytes are solutions that contain ions and allow the flow of electricity between the anode and cathode.

Corrosion Studies
Electrochemical consumables are employed in corrosion studies to investigate the degradation of metals and materials. These studies help researchers understand the mechanisms of corrosion and develop strategies to prevent or mitigate it. Electrochemical consumables used in corrosion studies include:
-
Electrodes: Electrodes are used to measure the electrical potential and current flow in corrosion experiments.
-
Electrolytes: Electrolytes are solutions that contain ions and allow the flow of electricity between the electrodes.
-
Corrosion Cells: Corrosion cells are专门 designed electrochemical cells used to simulate and study corrosion processes.
Chemical Analysis
Electrochemical consumables are utilized in various analytical techniques to determine the concentration or properties of substances in solution. These techniques include:
-
Cyclic Voltammetry: Cyclic voltammetry is an electrochemical technique that involves sweeping the potential of a working electrode and measuring the resulting current. It is used to study the redox behavior of substances and determine their electrochemical properties.
-
Potentiometry: Potentiometry is an electrochemical technique that involves measuring the potential difference between two electrodes in a solution. It is used to determine the concentration of ions in solution and study equilibrium reactions.
-
Amperometry: Amperometry is an electrochemical technique that involves measuring the current flow between two electrodes in a solution. It is used to study the kinetics of electrochemical reactions and determine the concentration of electroactive species in solution.
These are just a few examples of the many applications of electrochemical consumables in various industries and research fields. These specialized materials play a critical role in advancing our understanding of electrochemical processes and enabling the development of new technologies and products.
Two-Electrode and Three-Electrode Setups
Two-Electrode Setup
In a two-electrode setup, a single electrode serves as both the working electrode and the counter electrode. This setup is commonly used in simple electrochemical experiments, such as qualitative analysis and corrosion studies. The main advantage of the two-electrode setup is its simplicity. However, it has several disadvantages, including the inability to control the potential of the working electrode and the difficulty in separating the contributions of the working and counter electrodes to the overall current.
Three-Electrode Setup
In a three-electrode setup, a dedicated reference electrode is used to control the potential of the working electrode. This allows for more accurate and reproducible measurements. The counter electrode is used to complete the circuit and to provide a path for the current to flow. The three-electrode setup is the most common configuration used in electrochemical experiments.
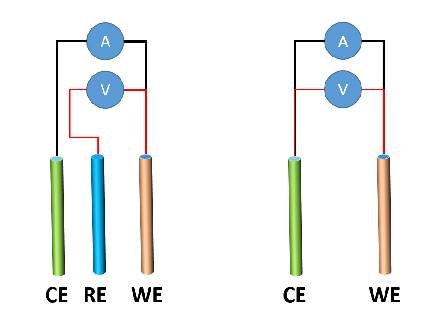
Advantages and Disadvantages of Two-Electrode and Three-Electrode Setups
The following table summarizes the advantages and disadvantages of two-electrode and three-electrode setups:
| Setup | Advantages | Disadvantages |
| Two-Electrode | Simple | Cannot control the potential of the working electrode | Difficult to separate the contributions of the working and counter electrodes to the overall current | | Three-Electrode | More accurate and reproducible measurements | More complex | Requires a dedicated reference electrode |
When to Use a Two-Electrode or Three-Electrode Configuration
The choice of whether to use a two-electrode or three-electrode configuration depends on the specific experiment being performed. In general, a three-electrode setup is preferred for quantitative analysis and mechanistic studies. A two-electrode setup may be used for qualitative analysis and corrosion studies.
Applications of Two-Electrode and Three-Electrode Setups
Two-electrode and three-electrode setups are used in a wide variety of electrochemical experiments. Some of the most common applications include:
- Cyclic voltammetry: This technique is used to study the electrochemical properties of a material by cycling the potential of the working electrode between two values.
- Linear sweep voltammetry: This technique is used to study the electrochemical properties of a material by sweeping the potential of the working electrode linearly from one value to another.
- Chronoamperometry: This technique is used to study the current-time response of a material to a step change in potential.
- Potentiometry: This technique is used to measure the potential of a material under equilibrium conditions.
Conclusion
Two-electrode and three-electrode setups are essential tools for electrochemical experiments. The choice of which configuration to use depends on the specific experiment being performed.
Conclusion
In conclusion, electrochemical consumables play a crucial role in the success of electrochemical experiments and applications. The choice of electrode materials and electrolytes must be carefully considered based on various factors, including reactivity, inertness, corrosion resistance, and compatibility with the specific application. Understanding the different types of electrodes and electrolytes, as well as the considerations for material selection, is essential for researchers and practitioners in the field of electrochemistry. By selecting appropriate electrochemical consumables, scientists can obtain accurate and reliable results, leading to advancements in research and technological developments.
Related Products
- Electrode Polishing Material for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Metal Disc Electrode Electrochemical Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
Related Articles
- Comprehensive Guide to Rotating Disk Electrode (RDE) in Electrochemical Studies
- Understanding Electrodeposition with Electrochemical Electrodes
- Electrode Materials for Rotating Ring-Disk Electrodes
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments


















