
Electrochemical Consumables
Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Item Number : ELEQ
Price varies based on specs and customizations
$49.90 / set
- Specifications
- 10ml ~ 1000ml
- Applicable temperature range
- 0 ~ 60℃
- Material
- Quartz glass + PTFE
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
Our quartz electrochemical cell is ideal for conducting electrochemical experiments due to its excellent corrosion resistance and complete specifications. We've carefully selected high-quality materials and ensured good sealing to guarantee a safe and durable product. Additionally, the cell can be customized to meet your specific needs.
Technical specifications
Quartz sealed electrolytic cell
| Specifications | 10ml ~ 1000ml |
| Applicable temperature range | 0 ~ 60℃ |
| Sealing form | thread + apron |
| Material | Quartz glass + PTFE |
| Opening hole of electrolytic cell | Three electrode holes (6mm), Two air holes (3mm), can be customized |
Quartz unsealed electrolytic cell
| Specifications | 10ml ~ 1000ml |
| Applicable temperature range | 0 ~ 60℃ |
| Material | Quartz glass + PTFE |
| Opening hole of electrolytic cell | Three electrode holes (6mm) |
Detail & Parts
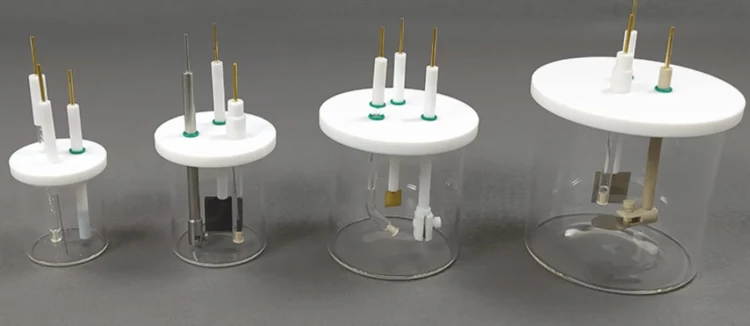




Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Are Electrolytic Cells Used For?
What Is The Difference Between Galvanic Cell And Electrolytic Cell?
What Is An Electrolytic Cell And How Does It Work?
What Are The Two Points Of Difference Between Electrochemical And Electrolytic Cells?
What Is The Example Of Electrolytic Cell?
Are Electrolytic Cells Spontaneous?
4.9 / 5
This Quartz electrolytic cell has been a game-changer in my laboratory. The quality is exceptional, and its durability has stood the test of time.
4.7 / 5
The Quartz electrolytic cell has exceeded my expectations. Its precision and accuracy have taken my experiments to the next level.
4.8 / 5
I'm thrilled with the Quartz electrolytic cell. The customizable options allowed me to tailor it to my specific needs, making it an invaluable tool in my laboratory.
4.6 / 5
The Quartz electrolytic cell has been a valuable addition to my lab. Its corrosion resistance and ease of use have made my experiments more efficient and productive.
4.8 / 5
The Quartz electrolytic cell is a testament to KINTEK SOLUTION's commitment to quality. Its construction is impeccable, and the results it delivers are consistently reliable.
4.7 / 5
The Quartz electrolytic cell is an absolute steal. Its affordability, combined with its exceptional performance, makes it an unbeatable value for money.
4.9 / 5
KINTEK SOLUTION's Quartz electrolytic cell is a marvel of engineering. Its technological advancements have revolutionized my electrochemical experiments, enabling me to achieve unprecedented results.
4.6 / 5
The Quartz electrolytic cell is a must-have for any laboratory. Its versatility and wide range of applications make it an indispensable tool for researchers across various disciplines.
4.7 / 5
The Quartz electrolytic cell has transformed my laboratory. Its speed and efficiency have significantly reduced my experimental turnaround time, allowing me to conduct more experiments in less time.
4.8 / 5
KINTEK SOLUTION's Quartz electrolytic cell is a testament to their customer-centric approach. The exceptional quality and performance of this product have made it an indispensable part of my laboratory.
4.9 / 5
The Quartz electrolytic cell is a game-changer for electrochemists. Its innovative design and user-friendly features make it an absolute pleasure to work with.
4.7 / 5
The Quartz electrolytic cell has been a revelation in my laboratory. Its accuracy and precision have enabled me to obtain consistent and reliable results, enhancing the quality of my research.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Electrolytic Electrochemical Cell for Coating Evaluation
Looking for corrosion-resistant coating evaluation electrolytic cells for electrochemical experiments? Our cells boast complete specifications, good sealing, high-quality materials, safety, and durability. Plus, they're easily customizable to meet your needs.

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.

PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Choose our PTFE Electrolytic Cell for reliable, corrosion-resistant performance. Customize specifications with optional sealing. Explore now.

H Type Electrolytic Cell Triple Electrochemical Cell
Experience versatile electrochemical performance with our H-type Electrolytic Cell. Choose from membrane or non-membrane sealing, 2-3 hybrid configurations. Learn more now.

Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
Looking for a high-quality gas diffusion electrolysis cell? Our liquid flow reaction cell boasts exceptional corrosion resistance and complete specifications, with customizable options available to suit your needs. Contact us today!

Flat Corrosion Electrolytic Electrochemical Cell
Discover our flat corrosion electrolytic cell for electrochemical experiments. With exceptional corrosion resistance and complete specifications, our cell guarantees optimal performance. Our high-quality materials and good sealing ensure a safe and durable product, and customization options are available.

Optical Water Bath Electrolytic Electrochemical Cell
Upgrade your electrolytic experiments with our Optical Water Bath. With controllable temperature and excellent corrosion resistance, it's customizable for your specific needs. Discover our complete specifications today.

Super Sealed Electrolytic Electrochemical Cell
Super-sealed electrolytic cell offers enhanced sealing capabilities, making it ideal for experiments that require high airtightness.

Electrode Polishing Material for Electrochemical Experiments
Looking for a way to polish your electrodes for electrochemical experiments? Our polishing materials are here to help! Follow our easy instructions for best results.

Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
High-quality graphite electrodes for electrochemical experiments. Complete models with acid and alkali resistance, safety, durability, and customization options.

Gold Electrochemical Sheet Electrode Gold Electrode
Discover high-quality gold sheet electrodes for safe and durable electrochemical experiments. Choose from complete models or customize to meet your specific needs.

Gold Disc Electrode
Looking for a high-quality gold disc electrode for your electrochemical experiments? Look no further than our top-of-the-line product.

RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
Elevate your electrochemical research with our Rotating Disk and Ring Electrodes. Corrosion resistant and customizable to your specific needs, with complete specifications.

Electrode Fixture for Electrochemical Experiments
Upgrade your experiments with our customizable Electrode Fixtures. High-quality materials, acid and alkali resistant, and safe and durable. Discover our complete models today.

Platinum Auxiliary Electrode for Laboratory Use
Optimize your electrochemical experiments with our Platinum Auxiliary Electrode. Our high-quality, customizable models are safe and durable. Upgrade today!

Rotating Platinum Disk Electrode for Electrochemical Applications
Upgrade your electrochemical experiments with our Platinum Disc Electrode. High-quality and reliable for accurate results.
Optical Window Glass Substrate Wafer Quartz Plate JGS1 JGS2 JGS3
The quartz plate is a transparent, durable, and versatile component widely used in various industries. Made from high-purity quartz crystal, it exhibits excellent thermal and chemical resistance.

Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
K9 glass, also known as K9 crystal, is a type of optical borosilicate crown glass renowned for its exceptional optical properties.

Button Battery Tablet Press Sealing Mold for Lab Use
The sealing die is essential for assembling button batteries, ensuring components like the anode, cathode, and electrolyte are securely enclosed.
Related Articles

Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
Explore the detailed workings, applications, and benefits of quartz electrolytic cells in various industries. Learn how these cells facilitate precise chemical reactions and their role in high-purity metal production.

The Art of the Empty Vessel: Preparing Quartz Electrolytic Cells for Absolute Precision
Reliable electrochemical data isn't just about the reaction; it's about the setup. Discover the systematic approach to preparing quartz cells for perfect fidelity.

The Geometry of Control: Why Millimeters Matter in Electrochemistry
Understanding the standard specifications of quartz electrolytic cells—Φ6.2mm and Φ3.2mm openings—and how they define experimental boundaries.

The Invisible Variable: Why Cell Geometry Defines Electrochemical Success
Discover how selecting the right quartz electrolytic cell volume and geometry impacts experimental accuracy. From standard 30ml units to custom designs.

The Glass Heart: Why Good Science Dies in Dirty Cells
The reliability of your electrolytic cell isn't just about chemistry; it's about discipline. Learn the systemic protocols for quartz and electrode maintenance.

The Architecture of Invisibility: Deconstructing the "All-Quartz" Cell
An engineering deep dive into the construction of electrolytic cells. Why material interfaces matter, and how to choose between quartz and glass for data integrity.

The Silent Dialogue: Mastering Control in Electrolytic Cells
Electrolysis is a non-spontaneous act requiring precise control. Learn to interpret the interplay of voltage, current, and physical phenomena for safer lab results.

The Glass Heart of the Experiment: Mastering the Electrolytic Cell
Master the art of handling electrolytic cells. Learn the critical balance between physical fragility, chemical safety, and operational precision.

The Architecture of Precision: Why Your Electrolytic Cell Specs Matter More Than You Think
Discover the critical logic behind electrolytic cell specifications. From aperture geometry to volume trade-offs, learn how the right vessel defines experimental success.

The Vessel of Truth: Why the Container Matters More Than the Chemistry
The success of an electrolytic experiment often hangs on the material of the cell body. Discover the trade-offs between Borosilicate, Quartz, and PTFE.

The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
Master the art of pre-use inspection for electrolytic cells. From physical integrity to electrode purity, learn why the invisible details dictate experimental safety.

The Glass Heart of the Experiment: Precision Through Systematic Care
Routine maintenance of double-layer electrolytic cells isn't just cleaning—it's calibration. Discover the systematic protocol for reproducible electrochemical data.