Definition and Basic Functions of Electrolytic Cell
Table of Contents
Understanding the role of electrical energy and chemical energy in an electrolytic cell
Electrochemical cell An electrochemical cell is a device capable of either generating electrical energy from chemical reactions or facilitating chemical reactions through the introduction of electrical energy.
Define Electrochemistry Electrochemistry is the study of chemical processes that cause electrons to move. It deals with the interaction between electrical energy and chemical change.
Example: Study of electrochemical cells comes in electrochemistry. It deals with cells that converts chemical energy to electrical energy.
Importance of Electrochemistry Electrochemistry deals with the relations between electrical and chemical phenomena. Electrochemical processes are used in various branches of industry. It is the most important process for the production of several chemicals. The production of various metals is based on electrochemical method. Hydrogen is manufactured by the electrolysis of water. It also plays a major role in the development of the electric automobile.
Description of the components of an electrolytic cell including electrodes and electrolyte
Electrolytic Cells are comprised of 3 essential components.

The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during an electrochemical reaction.
The Cathode is the positive or oxidizing electrode that acquires electrons from the external circuit and is reduced during the electrochemical reaction.
The Electrolyte is the medium that provides the ion transport mechanism between the cathode and anode of a cell. Electrolytes are often thought of as liquids, such as water or other solvents, with dissolved salts, acids, or alkalis that are required for ionic conduction. It should however be noted that many batteries including the conventional (AA/AAA/D) batteries contain solid electrolytes that act as ionic conductors at room temperature.
Considerations in selection of Cathode, Anode and Electrolyte
Desirable properties for anode, cathode, and electrolyte materials are noted below.
Explanation of the process of charge transfer between electrodes and ions
electrolytic cell, any device in which electrical energy is converted to chemical energy, or vice versa. Such a cell typically consists of two metallic or electronic conductors (electrodes) held apart from each other and in contact with an electrolyte (q.v.), usually a dissolved or fused ionic compound. Connection of the electrodes to a source of direct electric current renders one of them negatively charged and the other positively charged. Positive ions in the electrolyte migrate to the negative electrode (cathode) and there combine with one or more electrons, losing part or all of their charge and becoming new ions having lower charge or neutral atoms or molecules; at the same time, negative ions migrate to the positive electrode (anode) and transfer one or more electrons to it, also becoming new ions or neutral particles. The overall effect of the two processes is the transfer of electrons from the negative ions to the positive ions, a chemical reaction (see oxidation-reduction reaction). An example is the electrolysis of sodium chloride (common salt), forming sodium metal and chlorine gas; the energy required to make the reaction proceed is supplied by the electric current. Other common applications of electrolysis include electrodeposition for refining or plating of metals and the production of caustic soda.
What is an Electrolytic Cell? An electrolytic cell can be defined as an electrochemical device that uses electrical energy to facilitate a non-spontaneous redox reaction. Electrolytic cells are electrochemical cells that can be used for the electrolysis of certain compounds. For example, water can be subjected to electrolysis (with the help of an electrolytic cell) to form gaseous oxygen and gaseous hydrogen. This is done by using the flow of electrons (into the reaction environment) to overcome the activation energy barrier of the non-spontaneous redox reaction.


The three primary components of electrolytic cells are: Cathode (which is negatively charged for electrolytic cells) Anode (which is positively charged for electrolytic cells) Electrolyte The electrolyte provides the medium for the exchange of electrons between the cathode and the anode. Commonly used electrolytes in electrolytic cells include water (containing dissolved ions) and molten sodium chloride.
ELECTROLYTIC CELLS To define electrolytic cells, we need first to understand electrolysis. Electrolysis is a method that entails passing an electric current through a liquid containing ions, causing the compounds inside to disintegrate. This is used to isolate the metal from metallic elements, segregate other chemical substances (such as water) and electroplate metals, and recharge batteries. A complete circuit is essential to maintain an electrolytic process; we need to be able to draw electricity from the cell continually.
An electrolytic cell's cathode and anode are connected to a power source. These two stable electrodes and a fluid electrolyte solution are found in every electrolytic cell. The electrolyte solution conducts electricity since dissolved ions can move freely in the solution.
Electrolytic Cell And Its Components The electrolytic cell consists of three main components – battery, electrodes and electrolyte. Battery The battery functions as the power source. It provides the electrical energy to bring about the chemical change, i.e., the decomposition of the ionic compound. Electrodes Electrodes are electrical contacts that close or complete the electric circuit between the wires and the electrolyte. Examples of electrodes include carbon rods (graphite) and metal plates. The electrode on the left (refer to image above) that is connected to the positive terminal of the battery is the positive electrode or anode. The electrode on the right that is connected to the negative terminal of the battery is the negative electrode or cathode. Electrolyte The electrolyte is a substance that contains mobile ions when molten or in aqueous solution, to conduct electricity. Examples of electrolytes include molten sodium chloride, copper(II) sulfate solution and acids, such as dilute hydrochloric acid. Examples of non−electrolytes include sugar solution, ethanol, (molten) sulfur, as these are liquids or solutions that do not contain mobile ions to conduct electricity.

Applications Electrolytic cells are often used to decompose chemical compounds, in a process called electrolysis— with electro meaning electricity and the Greek word lysis means to break up. Important examples of electrolysis are the decomposition of water into hydrogen and oxygen, and bauxite into aluminum and other chemicals. Electroplating (e.g., of copper, silver, nickel, or chromium) is done using an electrolytic cell. Electrolysis is a technique that uses a direct electric current (DC).
Commercially, electrolytic cells are used in the electrorefining and electrowinning of several non-ferrous metals. Most high-purity aluminum, copper, zinc, and lead are produced industrially in electrolytic cells.
An electrolytic cell The three components of electrolytic cells are an electrolyte and two electrodes.
Electrolytes Electrolytes are the substances that give an electrically conducting solution when dissolved in polar solvents, such as water. This is because when the electrolyte is dissolved in the polar solvents, it breaks into cation and anions and gets distributed uniformly throughout the solution. These cations and anions under an electrical potential in the solution move to the electrode with an abundance of electrons and a deficit of electrons, respectively. This movement of cation and anions in the direction opposite to each other generates current and forms the electrolytic cells.
While salts, acids and bases form an electrolyte, few gases under certain conditions can also behave like an electrolyte, such as hydrogen chloride at high temperature and low pressure.
Chemical Reactions in Electrolytic Cell
Explanation of oxidation-reduction reaction in an electrolytic cell
In electrochemical cells, oxidation-reduction (redox) reactions take place. There are two types of electrochemical cells: galvanic (voltaic) cells, where spontaneous reactions occur, and electrolytic cells, where non-spontaneous reactions occur.
Both types of cells have electrodes where oxidation and reduction reactions occur. Oxidation happens at the electrode called the anode, and reduction occurs at the electrode called the cathode.
In an electrolytic cell, the anode is positive and the cathode is negative. This is because the anode attracts anions from the solution. On the other hand, in a galvanic cell, the anode is negatively charged, as the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The cathode of a galvanic cell is its positive terminal.
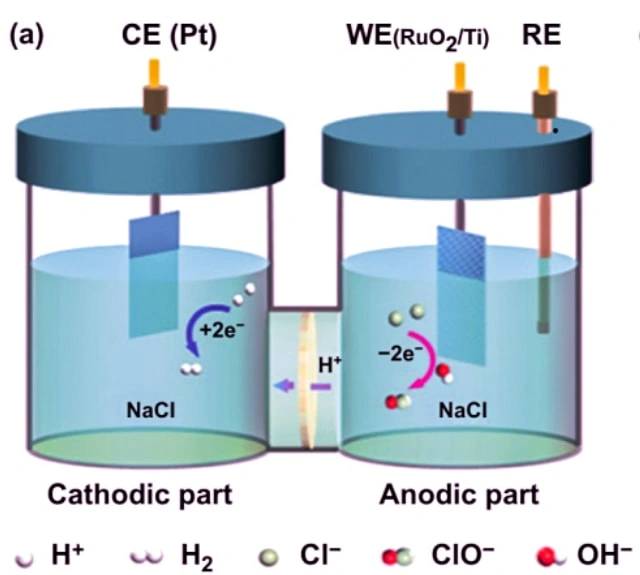
Example of electrolysis of sodium chloride
One example of an electrolytic cell is the electrolysis of sodium chloride. When sodium chloride is dissolved in water and subjected to electrolysis, the following reactions occur:
At the cathode: 2 H2O + 2 e- -> 2 OH- + H2
At the anode: 2 Cl- -> Cl2 + 2 e-
Overall reaction: 2 NaCl + 2 H2O -> 2 Na + Cl2 + H2 + 2 OH-
In this electrolytic cell, two inert electrodes are immersed in molten sodium chloride. When an electric current passes through the circuit, the cathode becomes rich in electrons and develops a negative charge. The positively charged sodium ions are attracted to the cathode, resulting in the formation of sodium metal. At the same time, chlorine atoms are attracted to the positively charged anode, leading to the formation of chlorine gas. The overall result is the production of sodium metal, chlorine gas, and aqueous sodium hydroxide.
Applications of Electrolytic Cells
Electrolytic cells have various applications:
- Production of oxygen gas and hydrogen gas from water.
- Extraction of aluminum from bauxite.
- Electroplating, which involves forming a thin protective layer of a specific metal on the surface of another metal.
- Electrorefining of non-ferrous metals.
- Electrolytic recovery processes.
- Industrial production of high-purity copper, high-purity zinc, and high-purity aluminum is almost always done through electrolytic cells.
![Application of electrolytic cells (high purity zinc, high purity aluminum, high purity copper)]()
Application of electrolytic cells (high purity zinc, high purity aluminum, high purity copper)
Electrolytic cells play a crucial role in various industries and processes, enabling the production of important chemicals and materials.
Applications of Electrolysis
Use of electrolysis in electrodeposition for refining or plating of metals
Electrolysis is widely used in the electrodeposition process for refining or plating metals. One practical application of electrolysis in this context is electro-cleaning. In electro-cleaning, the article to be cleaned, such as zinc or aluminum, is made the cathode, and a heavy current is passed through an electrolyte solution. Caustic soda and hydrogen are produced at the cathode, which effectively removes grease and impurities from the surface of the article. This process can be used for refining or plating metals, providing a clean and shiny appearance, protecting against corrosion, and replacing worn-out materials.
Production of caustic soda through electrolysis
Another significant application of electrolysis is in the production of chemicals, including caustic soda (NaOH) and chlorine gas, on a large scale. In this process, electrolysis is used to break down sodium chloride (NaCl) into its constituent elements, sodium (Na) and chlorine (Cl2). The reaction at the cathode involves the reduction of sodium ions (Na+) to metallic sodium (Na), while at the anode, chloride ions (Cl-) are oxidized to form chlorine gas (Cl2). The overall cell reaction is 2NaCl → 2Na + Cl2. Through this electrolytic process, large quantities of caustic soda and chlorine gas can be produced for various industrial applications.
Electrolysis in the extraction of metals
Electrolysis plays a crucial role in the extraction of metals from their ores. There are two primary methods of metal extraction using electrolysis. In the first method, the ore is treated with a strong acid to obtain a salt, and the resulting salt solution is electrolyzed to liberate the metal. In the second method, the ore is in a molten state and is directly electrolyzed in a furnace.
One example of metal extraction using electrolysis is the extraction of zinc. The zinc ore is treated with sulfuric acid to form zinc sulfate solution, which is then electrolyzed. In the electrolytic tank, aluminum cathodes and lead anodes are used. Zinc is deposited on the cathodes, while the sulfuric acid is regenerated at the anodes. This electrolytic process allows for the extraction of high-purity zinc.
Similarly, electrolysis is also used in the extraction of aluminum from bauxite. Bauxite, an aluminum ore, is dissolved in molten cryolite and subjected to electrolysis. The aluminum ions are reduced at the cathode, forming metallic aluminum, while oxygen gas is produced at the anode. This process enables the production of high-purity aluminum.

Overall, electrolysis finds practical applications in various industries for the extraction, refining, and plating of metals, as well as the production of chemicals such as caustic soda. By harnessing the power of electrolysis, businesses can achieve high-quality results and meet the demands of their respective industries.
Conversion of Chemical Energy to Electricity
Explanation of energy generation in reactions involving substances that generate energy
In electrochemical cells, chemical energy is converted into electrical energy through a process called electrolysis. Electrolysis is a redox reaction that occurs in an electrochemical cell, where chemical substances are decomposed at the electrodes. The products of electrolysis depend on the type of electrode and electrolyte used in the cell. This process is the basis for various applications in electrochemistry.
The field of electrochemistry involves the production of electricity from the energy released during spontaneous chemical reactions, as well as the use of electrical energy to drive non-spontaneous chemical changes. Redox reactions, which involve the transfer of electrons between species, are at the core of these processes. Many chemical and biological reactions are redox reactions, and they play a crucial role in obtaining energy for domestic, transport, or industrial purposes.
The energy generated in these reactions is used in a wide range of applications, including burning fuel for the digestion of food in animals, industrial processes, photosynthesis, extracting metals from ores, manufacturing important chemicals, and operating batteries and fuel cells.
Example of lead-acid storage battery
One example of the conversion of chemical energy to electricity is the lead-acid storage battery. This type of battery is commonly used as a power source in vehicles. It consists of multiple electrochemical cells connected together.

The lead-acid battery operates as both an electrolytic cell and a galvanic cell. When discharging, it acts as a galvanic cell, converting chemical energy into electrical energy. During the discharge process, lead dioxide, lead metal, and sulfuric acid react to form lead sulfate and water. The oxidation of lead to lead sulfate occurs at one electrode, while the reduction of lead dioxide to lead sulfate occurs at the other electrode. Electric charge is transported through the electrolyte by the migration of hydrogen ions.
This separation of oxidation and reduction processes creates a driving force, or a voltage, that causes electricity to flow through an external circuit connecting the two electrodes. Many other chemical combinations have been utilized in cells and batteries, each having its own specific reactions and characteristics.
Fuel cells
Fuel cells are another type of electrochemical cell that converts the chemical energy of fuel into electricity. These cells operate through an electrochemical reaction between hydrogen fuel and an oxidizing agent, typically oxygen. Unlike batteries, which generate energy from chemicals already filled within them, fuel cells require a continuous flow of oxygen and a supply of fuel to generate electricity.
Fuel cells have been used commercially by NASA to generate power for space capsules and satellites. They offer advantages in terms of thermodynamic efficiency, as they avoid Carnot cycle losses. However, current fuel cell technology still faces challenges in terms of overall efficiency and reliability. One limitation is the inability to use hydrocarbons directly, requiring a processing step to convert common fuels into hydrogen. Additionally, the catalysis of oxygen reduction is a complex process.

In summary, the conversion of chemical energy to electricity is a fundamental process in electrochemistry. It occurs through redox reactions in electrochemical cells, such as lead-acid storage batteries and fuel cells. These technologies have various applications, from powering vehicles to generating electricity for space exploration.
Alternative Cells for Generating Electricity
Understanding solar cells and the role of semiconductors and light absorption
- Amorphous silicon (A-Si) is a dominant thin-film PV material that has a light absorptivity about 40 times that of crystalline silicon.
- Cadmium telluride (CdTe) is another thin-film material with high light absorptivity. It can absorb 90% of the solar spectrum with just a 1mm thin film.
- Copper indium diselenide (CIGS) is a semiconductor material that has achieved high efficiency in PVs, with commercial modules reaching efficiencies of 14% or more.

Explanation of fuel cells and their use of oxidizing agents and reducing agents
- Fuel cells are electrochemical cells that react hydrogen fuel with oxygen or another oxidizing agent to convert chemical energy to electricity.
- They are different from batteries as they require a continuous source of fuel and oxygen to sustain the chemical reaction.
- Fuel cells can produce electricity continuously as long as fuel and oxygen are supplied.
- They are used for primary and backup power in various applications, including commercial, industrial, residential buildings, and remote areas.
- Fuel cells are also used to power fuel cell vehicles like forklifts, automobiles, buses, boats, motorcycles, and submarines.
- The global fuel cell market is expected to increase by 19.9% by 2030.
Other cells for generating electricity
- Solar cells generate electricity through the absorption of light by semiconductors.
- Fuel cells use chemical energy from fuel to generate electricity.
- Rechargeable cells can be used multiple times by recharging them.
- Non-rechargeable cells cannot be reused and need to be disposed of.
- Lead-acid/lead storage batteries are secondary cells used as a power source in vehicles.
- Thin-film cells, such as thin film solar cells, thin film transistors, and thin film batteries, offer improved efficiency, faster charging, and longer lifespan compared to conventional cells.
The field of alternative cells for generating electricity is constantly evolving. Researchers continue to improve the efficiency and properties of thin film materials, while industry experts work towards reducing costs and scaling up production.
If you are interested in this product you can browse our company website:https://kindle-tech.com/products/h-type-electrolytic-cell-h-type-triple, we always insist on the principle of quality first. During the production process, we strictly control every step of the process, using high quality materials and advanced production technology to ensure the stability and durability of our products. to ensure that their performance meets the highest standards. We believe that only by providing customers with excellent quality can we win their trust and long-term cooperation.
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- Platinum Sheet Electrode for Laboratory and Industrial Applications
Related Articles
- Overcoming Challenges with H-Type Electrolytic Cell Operation
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Electrochemistry The Science Behind Electrochemical Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
- The Unseen Variable: Mastering the Electrolytic Cell Inspection

















