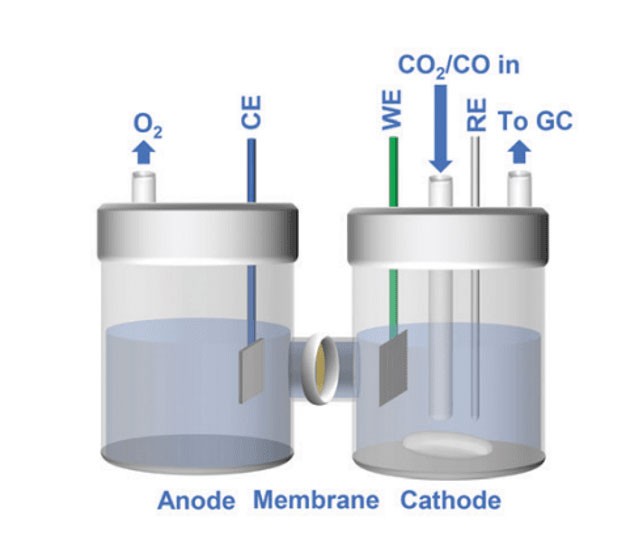
Introduction to Quartz Electrolytic Cells
Quartz electrolytic cells are pivotal in transforming electrical energy into chemical energy, playing a crucial role in various industrial processes. These cells are characterized by their use of quartz as a key material, which not only withstands high temperatures but also maintains chemical inertness. At their core, quartz electrolytic cells consist of electrodes and electrolytes, which together facilitate precise oxidation and reduction reactions. This introduction sets the stage for a deeper exploration into the mechanisms, applications, and advantages of quartz electrolytic cells, highlighting their significance in industries ranging from metal production to pharmaceuticals.
Mechanism of Action in Quartz Electrolytic Cells
Quartz electrolytic cells are specialized devices used in various industrial processes, particularly in the electrorefining and electrowinning of non-ferrous metals such as aluminum, copper, zinc, and lead. These cells are designed to facilitate the migration of ions and the efficient conduction of oxidation and reduction reactions through the use of a quartz structure and a carefully chosen electrolyte medium.
Ion Migration and Electrode Roles
In a quartz electrolytic cell, the process begins when an external voltage is applied to the system. This voltage drives the migration of ions within the electrolyte. Positive ions, or cations, are attracted to the negatively charged cathode, while negative ions, or anions, move towards the positively charged anode. This movement of ions is crucial for the conduction of electricity and the initiation of chemical reactions.
At the cathode, reduction reactions occur. Here, cations gain electrons and are deposited onto the cathode surface. For example, in the electrorefining of copper, copper ions (Cu²⁺) gain electrons to form pure copper metal, which is then deposited onto the cathode. This process is essential for obtaining high-purity metals used in various industrial applications.
Conversely, at the anode, oxidation reactions take place. Anions lose electrons and release other ions or molecules. In the case of copper electrorefining, the anode is made of impure copper. As the copper dissolves, it releases impurities into the electrolyte, which are then removed, leaving behind pure copper at the cathode.
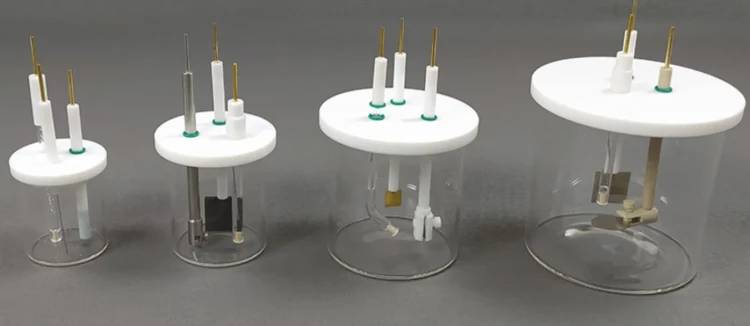
Importance of the Electrolyte Medium
The electrolyte medium in a quartz electrolytic cell plays a pivotal role in the efficiency and effectiveness of the reactions. The electrolyte must be a good conductor of ions and must be stable under the conditions of the reaction. Common electrolytes include aqueous solutions of salts, acids, or bases, as well as molten salts.
The choice of electrolyte depends on the specific requirements of the reaction, such as the desired pH, the concentration of ions, and the temperature at which the reaction occurs. For instance, in the production of high-purity aluminum, a molten mixture of cryolite (Na₃AlF₆) and alumina (Al₂O₃) is used as the electrolyte. This mixture has a low melting point and is highly conductive, making it ideal for the electrolytic reduction of alumina to aluminum.
Quartz Structure and Its Advantages
The use of quartz in electrolytic cells provides several advantages. Quartz is an excellent insulator, which helps in maintaining the integrity of the electrical field within the cell. Additionally, quartz is resistant to chemical corrosion, ensuring that the cell can operate under harsh conditions without degradation.
The quartz structure also allows for precise control over the distribution of ions and the flow of electricity. This precision is crucial for optimizing the efficiency of the reactions and minimizing energy losses. Furthermore, the transparency of quartz allows for visual monitoring of the reactions, which is beneficial for process control and troubleshooting.
Applications in Metal Purification and Electroplating
Quartz electrolytic cells are widely used in the purification of metals and in electroplating processes. In metal purification, such as the electrorefining of copper, the impure metal is used as the anode, and a pure metal is deposited onto the cathode. This process removes impurities and results in high-purity metal suitable for various industrial applications.
In electroplating, quartz electrolytic cells are used to deposit a thin layer of a desired metal onto a substrate. This process enhances the substrate's properties, such as its appearance, durability, and resistance to corrosion. Electroplating is commonly used in the automotive, aerospace, and electronics industries.
Conclusion
In summary, quartz electrolytic cells are essential tools in the production of high-purity metals and in various electrochemical processes. Their mechanism of action involves the migration of ions, the role of electrodes in facilitating oxidation and reduction reactions, and the importance of the electrolyte medium. The use of quartz provides several advantages, including insulation, corrosion resistance, and precise control over the reactions. These cells are widely used in metal purification and electroplating, contributing to the advancement of various industrial applications.
Applications of Quartz Electrolytic Cells
Quartz electrolytic cells play a crucial role in various industrial applications, particularly in the production of high-purity metals, water electrolysis, and the pharmaceutical industry. These cells are designed to withstand high temperatures and corrosive environments, making them ideal for processes that require precision and reliability.
Production of High-Purity Metals
One of the primary applications of quartz electrolytic cells is in the production of high-purity metals such as aluminum and copper. The electrolytic process involves the use of a direct current to drive a non-spontaneous chemical reaction. In the case of aluminum production, the raw material, bauxite, is dissolved in a molten cryolite bath. The aluminum ions are then reduced at the cathode, resulting in the deposition of pure aluminum. This process is highly energy-intensive but ensures a purity level of up to 99.99%.
Similarly, high-purity copper is produced through a process known as electrorefining. In this process, impure copper is used as the anode, and as the current is passed through the electrolytic cell, pure copper ions are deposited onto the cathode. The impurities either remain in the electrolyte or form a separate sludge at the bottom of the cell, ensuring the purity of the final product.
Water Electrolysis
Quartz electrolytic cells are also extensively used in water electrolysis, a process that splits water into its constituent elements, hydrogen and oxygen. This process is particularly useful in industries that require large quantities of these gases, such as the chemical and pharmaceutical industries. The electrolysis of water involves passing an electric current through water containing dissolved ions (typically from a salt or acid). The hydrogen ions (H+) migrate to the cathode, where they are reduced to hydrogen gas, while the hydroxide ions (OH-) migrate to the anode, where they are oxidized to oxygen gas.

Pharmaceutical Industry
In the pharmaceutical industry, quartz electrolytic cells are used in the production of various chemicals and compounds. For instance, the electrolysis of sodium chloride solution (brine) is used to produce chlorine gas and sodium hydroxide (caustic soda), both of which are essential raw materials in the synthesis of many pharmaceuticals. The precise control over the electrolytic process provided by quartz cells ensures the high purity and consistency of these chemicals, which are critical for pharmaceutical applications.
Electroplating and Surface Treatment
Another significant application of quartz electrolytic cells is in electroplating and surface treatment processes. Electroplating involves depositing a thin layer of a specific metal onto the surface of another metal to enhance its properties, such as corrosion resistance, wear resistance, or aesthetic appeal. Quartz cells provide a stable and controlled environment for these processes, ensuring uniform and high-quality coatings.
Energy Consumption and Efficiency
The energy consumption in electrolytic processes varies depending on the metal being produced. For example, the electrolytic production of aluminum requires approximately 13-15 kWh of electricity per kilogram of metal. In contrast, copper refining through electrolysis consumes between 150 to 300 kWh per tonne of refined copper. Despite the high energy requirements, the efficiency and purity of the final products make these processes indispensable in modern industry.
In conclusion, quartz electrolytic cells are vital in various industrial sectors, offering precise control and high efficiency in the production of high-purity metals, water electrolysis, and pharmaceutical chemicals. Their ability to operate in harsh environments and their durability make them an essential tool in modern manufacturing and processing industries.
Advantages of Using Quartz in Electrolytic Cells
Quartz, a form of silica (SiO2), is renowned for its unique properties that make it an ideal material for use in electrolytic cells. These cells are crucial in various industrial processes, including the production of chemicals, metals, and semiconductors. The selection of quartz over other materials like glass is primarily due to its superior resistance to high temperatures, chemical inertness, and excellent electrical properties.
High Temperature Resistance
Quartz can withstand temperatures up to 1100°C, significantly higher than glass, which softens at around 700°C. This high-temperature resistance is vital in electrolytic cells where temperatures can rise considerably due to the electrical currents and chemical reactions involved. The ability of quartz to maintain its structural integrity under such conditions ensures the longevity and efficiency of the electrolytic process.
Chemical Inertness
Quartz is highly resistant to most chemicals, including acids, alkalis, and salts. This chemical inertness is particularly beneficial in electrolytic cells, where the environment is often highly corrosive due to the electrolytes used. By using quartz, the risk of chemical reactions that could degrade the cell's components is minimized, thereby enhancing the cell's durability and performance.

Electrical Properties
Unlike glass, which is a good insulator, quartz is an excellent conductor of electricity. This property is crucial in electrolytic cells where the efficient conduction of electricity is essential for the electrolytic process to occur effectively. Quartz's high dielectric strength and low electrical loss make it an ideal material for electrodes and other electrical components within the cell.
Purity and Transparency
Quartz is highly pure, with a SiO2 content of at least 99.9%. This high purity is essential in industries such as semiconductor manufacturing, where even trace amounts of impurities can adversely affect the process. Quartz's transparency from the ultraviolet to the infrared spectrum also allows for better monitoring and control of the electrolytic process, ensuring higher quality outputs.
Mechanical and Optical Properties
Quartz exhibits exceptional mechanical properties, including high rigidity, elasticity, and resistance to shock. These properties ensure that quartz components can withstand the mechanical stresses encountered in electrolytic cells without compromising their structural integrity. Additionally, quartz's excellent optical transmission properties allow for precise monitoring and adjustment of the electrolytic process, contributing to higher efficiency and accuracy.
Applications in Electrolytic Cells
The unique properties of quartz make it suitable for a wide range of applications in electrolytic cells. Quartz tubes and rods, for instance, are used in semiconductor manufacturing for cleaning baths post-etching and machining, and in tubes undergoing heat treatments. In laboratories, quartz is used in sight gages, optics, and various industrial processes where its shock resistance and chemical inertness are invaluable.
In conclusion, the use of quartz in electrolytic cells leverages its superior properties, including high-temperature resistance, chemical inertness, excellent electrical properties, and mechanical and optical advantages. These characteristics not only enhance the performance and longevity of electrolytic cells but also contribute to the production of high-quality outputs in various industrial applications.
Comparison with Other Types of Electrolytic Cells
Electrolytic cells are a fundamental type of electrochemical cell, differing significantly from galvanic cells in their setup, function, and typical uses. Understanding these differences is crucial for selecting the appropriate cell type for specific applications in research and industry.
Key Differences Between Electrolytic and Galvanic Cells
-
Energy Conversion:
- Galvanic Cells: These cells convert chemical energy into electrical energy spontaneously. They are driven by redox reactions that occur naturally, producing a flow of electrons from the anode to the cathode through an external circuit. Examples include batteries and fuel cells.
- Electrolytic Cells: In contrast, electrolytic cells require an external electrical energy source to drive non-spontaneous chemical reactions. They are used in processes like electrolysis, where electrical energy is used to decompose compounds or drive reactions in a desired direction.
-
Spontaneity of Reactions:
- Galvanic Cells: The reactions in galvanic cells are spontaneous, meaning they occur without the need for an external energy source once the cell is set up.
- Electrolytic Cells: The reactions in electrolytic cells are non-spontaneous and require the input of electrical energy to proceed.
-
Electrode Polarities:
- Galvanic Cells: In galvanic cells, the anode is the negative electrode where oxidation occurs, and the cathode is the positive electrode where reduction occurs.
- Electrolytic Cells: The polarities are reversed in electrolytic cells. The anode is positive, and the cathode is negative, reflecting the need for an external power source to force electrons against their natural flow.

Typical Uses of Electrolytic and Galvanic Cells
- Galvanic Cells: Commonly used in everyday devices such as batteries (e.g., alkaline, lithium-ion), fuel cells, and solar cells. They are essential for portable electronic devices, providing a reliable source of electrical energy through spontaneous chemical reactions.
- Electrolytic Cells: Used in industrial processes such as electroplating (coating one metal onto another), water electrolysis (producing hydrogen and oxygen), and metal refining (e.g., copper refining). They are also crucial in the production of chemicals like sodium hydroxide and chlorine through the electrolysis of brine.
Structural Differences
- Galvanic Cells: Typically consist of two different electrolyte solutions in separate containers, connected by a salt bridge to maintain electrical neutrality. The electrodes (anode and cathode) are immersed in these solutions, and an external circuit allows the flow of electrons.
- Electrolytic Cells: Often have a simpler structure with both electrodes immersed in a single electrolyte solution. The external power source is connected to the electrodes to drive the electrolytic process.
Conclusion
While both electrolytic and galvanic cells are types of electrochemical cells and involve redox reactions, they serve fundamentally different purposes due to the nature of their reactions and the energy conversions they facilitate. Understanding these differences helps in selecting the appropriate cell type for specific applications, whether it be for generating electrical energy spontaneously or driving chemical reactions through the input of electrical energy.
Conclusion
In conclusion, quartz electrolytic cells stand out as critical components in modern industrial processes, leveraging their unique properties to facilitate precise chemical reactions and high-purity metal production. Their ability to withstand high temperatures and chemical inertness positions them as superior alternatives in various applications, from water electrolysis to pharmaceutical production. Despite challenges such as maintenance and electrode degradation, ongoing research promises innovations that will enhance their efficiency and expand their utility. As industries continue to evolve, the role of quartz electrolytic cells will undoubtedly grow, underscoring their significance in advancing technological and chemical processes.
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Side Window Optical Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
- Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- The Architecture of Invisibility: Deconstructing the "All-Quartz" Cell
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- The Vessel of Truth: Why the Container Matters More Than the Chemistry
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Art of the Empty Vessel: Preparing Quartz Electrolytic Cells for Absolute Precision