Definition and Function of an Electrode
Table of Contents
Description of an electrode
An electrode is a point where current enters and leaves the electrolyte. It is a conductor used to make a junction with a nonmetallic part of a circuit. Electrodes can be made of materials such as gold, platinum, carbon, graphite, or metal. They serve as the surface for oxidation-reduction reactions in electrochemical cells. There are different types of electrodes, including anode and cathode.
Role of an electrode in an electrochemical cell
Electrodes are essential components of electrochemical cells. They transport electrons produced in the cell from one half-cell to another, creating an electrical charge. The cathode is the electrode where current leaves the cell, while the anode is the electrode where current enters. The electrolyte in the cell acts as a conduit for electron flow between the cathode and anode. The cell potential is calculated based on a standard electrode system with a reference potential of 0 volts.

Difference between an anode and a cathode
The anode and cathode have different roles in an electrochemical cell. The cathode is negatively charged in electrolytic cells, and a reduction reaction occurs at this electrode. Electrons move into the cathode during the cell's operation. On the other hand, the anode is positively charged in electrolytic cells, and an oxidation reaction occurs at this electrode. Electrons move out of the anode during the cell's operation. It's important to note that the roles of anode and cathode can change depending on the direction of electron flow.
In summary, electrodes are conductors used in electrochemical cells to facilitate electron transfer. They play a crucial role in the functioning of these cells and are classified as anodes or cathodes based on the type of chemical reaction that occurs. Understanding the roles and functions of electrodes is essential in the study of electrochemistry.
Mechanics of an Electrode
Process of oxidation and reduction at the electrode surface
An electrode is a metal surface where oxidation-reduction equilibrium is established between the metal and the solution it is placed in. The electrode can be either an anode or a cathode.
-
Anode: The anode receives current or electrons from the electrolyte mixture, causing it to become oxidized. When atoms or molecules come close to the surface of the anode, the solution donates electrons, resulting in the formation of positive ions.
-
Cathode: The cathode, on the other hand, releases electrons into the solution, leading to reduction.
An electrode should be stable and resist corrosion, except in cases where the electrode is sacrificial or used for specific processes. Mechanical action can cause degradation of electrodes, such as the release of graphite particulates, which may require filtration. Swelling of the electrode can also be problematic with certain materials.
Role of anode and cathode in these processes
Anodes and cathodes play crucial roles in electrochemical systems, such as batteries, fuel cells, photovoltaic cells, electrolytic cells, and diodes.

-
Anode: The anode refers to the electrode where oxidation takes place or where electrons flow out. It is the terminal or conductor where electrons leave the electrochemical cell, causing oxidation to occur. In primary cells, the anode is fixed and cannot be recharged, while in secondary cells, the anode can change polarity depending on the direction of current.
-
Cathode: The cathode refers to the electrode where reduction takes place or where electrons flow in. Electrons enter the cell at the cathode and are involved in reduction reactions. Similar to the anode, the cathode can also change polarity in secondary cells.
Corrosion in electrodes can be severe, especially when there are fluctuating potentials in the electrolyte or when different metals are used as the anode and cathode. However, inhibitors and other preventive methods can help reduce electrode attacks.
Electrolysis is a process that occurs in electrolytic cells, where electrical energy is used to perform non-spontaneous chemical reactions. Oxidation occurs at the anode (positive plate), while reduction occurs at the cathode (negative plate).
Understanding the mechanics of an electrode, including the processes of oxidation and reduction, as well as the roles of the anode and cathode, is crucial in various electrochemical applications.
Material Composition of an Electrode
Requirements for an electrode material
The efficiency of electrochemical cells is determined by the physical properties of the electrodes. The material composition of an electrode plays a crucial role in its performance. The main requirement for an electrode material is conductivity. Any conducting material such as metals, semiconductors, graphite, or conductive polymers can be used as an electrode. In many cases, electrodes consist of a combination of materials, each with a specific task. These materials include:
- Active materials: These are the particles that undergo oxidation or reduction in the electrode.
- Conductive agents: They improve the conductivity of the electrode.
- Binders: They are used to contain the active particles within the electrode.
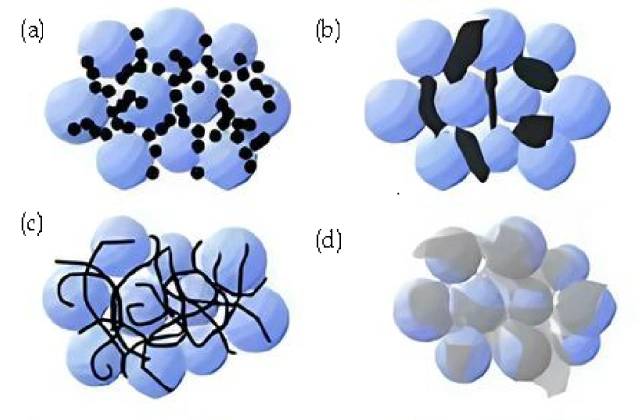
![Summary of conductive agents (a. Carbon black, rigid nanoparticles; point-to-point contact. b. Conductive graphite, rigid micron particles; point-to-point contact. c. Carbon nanotubes, flexible; line and point contact d. Graphite, flexible sheet; surface and point contact. )]()
Summary of conductive agents (a. Carbon black, rigid nanoparticles; point-to-point contact. b. Conductive graphite, rigid micron particles; point-to-point contact. c. Carbon nanotubes, flexible; line and point contact d. Graphite, flexible sheet; surface and point contact. )
In addition to conductivity, other important properties of electrodes include electrical resistivity, specific heat capacity, electrode potential, and hardness. The cost of the material is also a significant factor to consider for technological applications.
Examples of inert and reactive electrodes
An electrode can be either inert or reactive, depending on its involvement in the oxidation-reduction (redox) reaction. Inert electrodes do not participate in the reaction and are chemically unreactive. Examples of inert electrodes include graphite (carbon), platinum, gold, and rhodium. These materials are used when the electrode's sole purpose is to facilitate the flow of current in the electrochemical cell.
On the other hand, reactive electrodes actively participate in the redox reaction. They undergo oxidation or reduction along with the reactants. For example, a magnesium electrode is an active electrode because it participates in the reaction. The choice between inert and reactive electrodes depends on the specific requirements of the electrochemical process.
Role of the electrode in the reaction
The electrode serves as a platform for the redox reaction to occur. In some cases, solid forms of the reactants are used as the electrodes. For example, in a copper-silver electrochemical cell, copper and silver are both the reactants and the electrodes. The reactant-electrodes facilitate the transfer of electrons and ions during the redox reaction.
Alternatively, in reactions that require an inert electrode, a metal that does not participate in the reaction is used. An example of this is platinum in the Standard Hydrogen Electrode (SHE) reaction. The inert electrode allows the flow of current without interfering with the chemical reaction.
The choice of electrode material is crucial in achieving optimal yields and selectivity in electrochemical processes. The material's properties influence the kinetics and thermodynamics of electron transfer and can determine the success or failure of a transformation. Considerations such as cost, stability, and manipulability into various forms also play a role in selecting the appropriate electrode material.
The material composition and internal structure of an electrode are essential factors that determine its performance. The combination of active materials, conductive agents, and binders in an electrode slurry enhances its conductivity and functionality. The mixture is then coated onto a conductor, which acts as the current collector in the electrochemical cell.
In summary, the material composition of an electrode, whether inert or reactive, significantly impacts its efficiency and functionality in electrochemical processes. The choice of electrode material should be based on the specific requirements of the application, considering factors such as conductivity, stability, and cost.
Examples of Electrodes
Examples of commonly used inert and reactive electrodes
The distinction can be made between active electrodes and inert electrodes. For example, a magnesium electrode is usually an active electrode because it participates in the oxidation-reduction (redox) reaction. A platinum electrode is usually an inert electrode because it does not participate in the oxidation-reduction reaction. An inert electrode is chemically unreactive and is only present so that current can flow through the electrochemical cell.
Electrode Examples in Analytical Chemistry
Examples of typical materials used for electrodes in analytical chemistry are amorphous carbon, gold, and platinum. Glass electrodes are often used in pH measurements; in this application, the glass is chemically doped to be selective to hydrogen ions.
Electrode Examples in Batteries
Batteries contain a variety of electrodes, depending on the battery type.
- Lead-acid batteries are based on lead electrodes.
- Zinc-carbon batteries are made with zinc and amorphous carbon electrodes.
- Lithium polymer batteries have electrodes made of a solid polymer matrix within which lithium ions can move and act as charge carriers.
![Lead-acid batteries, zinc carbon batteries and lithium polymer batteries]()
Lead-acid batteries, zinc carbon batteries and lithium polymer batteries
Electrode Examples in Electrolysis
Electrical energy can be used to convert salts and ores to metals.
- In the Hall-Heroult process to extract aluminum metal from aluminum oxide, the anode and cathode are both made of graphite.
- Sodium metal is produced by electrolysis using a carbon anode and an iron cathode.
Inert Electrodes
A metal that doesn't interfere or participate in any chemical reactions is known as an inert electrode. However, it is still used to transfer electricity by passing electrons through the solution instead of exchanging ions.
Examples of inert electrodes include graphite, platinum, gold, and rhodium.
Electrodes in Quantitative Analysis
In potentiometric analysis, an indicator electrode responds to differences in the analyte's activity or "effective concentration". This simplicity makes potentiometry an economical technique compared to atomic spectroscopy or ion chromatography. These procedures can be classified based on the aspects of the cell that are controlled.
Uses of Electrodes
Electrodes are used to provide current through nonmetal objects to alter them in numerous ways and to measure conductivity for various purposes. Some examples include:

- Electrodes for fuel cells
- Electrodes for medical purposes, such as EEG (for recording brain activity), ECG (recording heart beats), ECT (electrical brain stimulation), defibrillator (recording and delivering cardiac stimulation)
- Electrodes for electrophysiology techniques in biomedical research
- Electrodes for execution by the electric chair
- Electrodes for electroplating
- Electrodes for arc welding
- Electrodes for cathodic protection
- Electrodes for grounding
- Electrodes for chemical analysis using electrochemical methods
- Nanoelectrodes for high-precision measurements in nanoelectrochemistry
- Inert electrodes for electrolysis (made of platinum)
- Membrane electrode assembly
- Electrodes for Taser electroshock weapon
Standard Hydrogen Electrode (SHE)
Definition and importance of SHE
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use as a reference for all half-cell potential reactions. The value of the standard electrode potential is zero, making it the basis for calculating cell potentials using different electrodes or concentrations. Having a common reference electrode like the SHE is crucial for accurate measurements and comparisons in electrochemical experiments.
Material composition and reaction process of SHE
The SHE is composed of a 1.0 M H+(aq) solution containing a square piece of platinized platinum. The platinum is connected to a platinum wire, allowing for electron exchange. Inside a glass tube, hydrogen gas is passed through and into the solution, resulting in the following reaction:
2H+(aq) + 2e- ⇌ H2(g)
This equilibrium between hydrogen ions and hydrogen gas establishes the reference potential of the SHE.

Challenges in setting up and use of SHE
Setting up and using the SHE can present some challenges. One challenge is ensuring the stability of the reference electrode over time and with changing temperatures. The components of the SHE must be stable and provide fixed, reproducible electrode potentials.
Another challenge lies in the construction of the reference electrode. The SHE consists of a glass jacket with a small inlet at the top and multiple outlets at the bottom. Inside the glass jacket, there is a glass tube sealed at both ends, containing the platinized platinum wire and a platinized platinum plate at the lower end. The glass jacket and tube are immersed in a vessel containing 1 M HCl solution. The proper construction of these components is essential for the accurate functioning of the SHE.
In summary, the Standard Hydrogen Electrode is a vital tool in electrochemistry, serving as a reference for measuring and comparing electrode potentials. Its construction and use require careful attention to ensure stability and accuracy in experimental measurements.
Three Electrode System
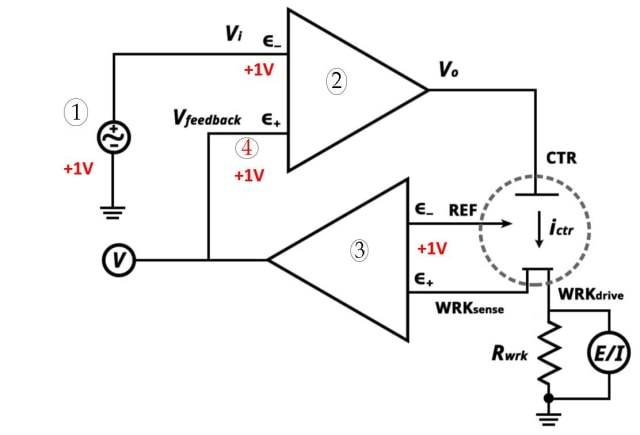
The three electrode system is an essential component in voltammetry. It consists of three electrodes: the working electrode, reference electrode, and auxiliary electrode. Each electrode plays a specific role in the system.
Description of the three electrode system
The three electrode system is used in electroanalytical chemistry to perform voltammetric analysis. It allows for the measurement and control of current flow in an electrochemical cell. The system consists of the following electrodes:
-
Working electrode: The working electrode is responsible for transporting electrons to and from the substances present in the cell. It facilitates the electrochemical reactions that occur during voltammetry.
-
Reference electrode: A reference electrode has an established electrode potential. It can be used as a half cell in an electrochemical cell. By comparing the potential of the working electrode with the reference electrode, the electrode potential of the working electrode can be determined.
-
Auxiliary electrode: The auxiliary electrode ensures that current does not pass through the reference cell. It balances the current with that of the working electrode. The auxiliary electrode is also known as the counter electrode.
![Three-electrode system (1. Potentiostatic set point 2. High Gain Op-Amp 3. Electrometer 4. Very close)]()
Three-electrode system (1. Potentiostatic set point 2. High Gain Op-Amp 3. Electrometer 4. Very close)
Roles of the working, reference, and auxiliary electrodes
In the three electrode system, each electrode has a specific role to play:
-
The working electrode facilitates the electrochemical reactions by transporting electrons to and from the substances in the cell.
-
The reference electrode provides a known electrode potential against which the potential of the working electrode can be measured. It acts as a point of reference for determining the electrode potential of the working electrode.
-
The auxiliary electrode, also known as the counter electrode, ensures that current flows through the electrochemical cell without passing through the reference electrode. It balances the current with that of the working electrode.
The three electrode system offers distinct advantages over two-electrode setups. It allows for the isolation of the working electrode, enabling the study of specific reactions with accuracy and confidence. This setup is commonly used in electrochemical experimentation.
Two-electrode setups, on the other hand, are used in cases where the measurement of the whole cell voltage is significant, such as in electrochemical energy devices like batteries, fuel cells, and supercapacitors. It can also be used when the counter-electrode potential is expected to remain stable throughout the experiment.
Overall, the three electrode system is a crucial tool in voltammetry, allowing for precise measurement and control of electrochemical reactions.
Examples of Reference Electrodes
Description and process of the Calomel electrode
The calomel electrode consists of a glass tube with a sidearm. At the bottom of the tube, there is pure mercury with a platinum wire sealed into it for electrical connections. Above the mercury, there is a paste of mercurous chloride (calomel) in mercury. The rest of the tube is filled with a saturated KCl solution. The sidearm is used for dipping it into any solution used for coupling the calomel electrode.

Working: The calomel electrode can act as a negative electrode, and two reactions are possible depending on the nature of the other electrode it is coupled with. When acting as a negative electrode, the following reactions occur:
- 2 Hg(l) → 2 Hg+ + 2 e–
- 2 Hg+ + 2 Cl– → Hg2Cl2(s)
The net oxidation reaction is the formation of mercurous chloride.
Advantages of Calomel Electrode:
- Relatively easier to make and maintain compared to the Standard Hydrogen Electrode (SHE)
- Composed of solid paste and liquid mercury, making it convenient to use
- Does not require a separate salt bridge as it already contains a side tube with KCl solution
- Potential does not change appreciably with time and slight temperature changes
Disadvantages of Calomel Electrode:
- Compensation for potential is necessary when measuring half-cell potentials
- Cannot be used in the measurement of potentials where K+ and Cl– ions interfere
- Oxidation potential depends on the concentration of KCl, so changes in concentration can affect the electrode's potential.
Description and process of the Silver-Silver Chloride electrode
The Silver-Silver Chloride electrode is widely used as a reference electrode due to its affordability and lower toxicity compared to the Calomel electrode. This electrode consists of solid silver and its precipitated salt, AgCl. It is made by coating a wire of solid silver with AgCl and immersing it in a tube of KCl and AgCl solution.

Working: The Silver-Silver Chloride electrode allows ions to be formed and electrons to flow in and out of the electrode system. It can act as a reference electrode when coupled with another electrode.
Advantages of Silver-Silver Chloride Electrode:
- Inexpensive and widely available
- Less toxic compared to the Calomel electrode
- Easy to set up and reproduce
- Convenient and easy to transport
- Compact and smaller in size, requiring little space
- No separate salt bridge is required as it already contains a side tube with KCl solution
- Potential remains relatively stable over time and with slight temperature changes
Reference electrodes are essential in electrochemical analysis as they provide a stable and constant potential for sense measurements. The Calomel electrode and Silver-Silver Chloride electrode are two examples commonly used in aqueous electrochemistry. They have their advantages and disadvantages, and their suitability depends on the specific application and requirements.
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Flat Corrosion Electrolytic Electrochemical Cell
- Gold Electrochemical Sheet Electrode Gold Electrode
Related Articles
- Understanding Saturated Calomel Reference Electrodes: Composition, Uses, and Considerations
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success
- Advanced Techniques in Coating Evaluation Using Electrolytic Cells
- The Glass Heart: Why Good Science Dies in Dirty Cells
- Understanding Flat Corrosion Electrolytic Cells: Applications, Mechanisms, and Prevention Techniques