Table of Contents
Introduction to Electrochemical Cells
Electrochemical cells are at the heart of energy storage and conversion. They harness chemical reactions to generate electricity or utilize electricity to drive chemical reactions. These cells are essential components in various electrochemical systems, including batteries, fuel cells, and electroplating, and are vital for advancements in energy technologies, electric vehicles, and electrochemical engineering.
Types of Electrochemical Cells
Electrochemical cells are devices that convert chemical energy into electrical energy (voltaic cells) or vice versa (electrolytic cells). They consist of two electrodes, a cathode and an anode, immersed in an electrolyte solution.
Voltaic Cells
Also known as galvanic cells, voltaic cells generate electricity from spontaneous chemical reactions. In a voltaic cell, the anode is the negative electrode where oxidation occurs, releasing electrons. These electrons flow through an external circuit to the cathode, the positive electrode, where reduction occurs. The spontaneous nature of the reaction drives the flow of electrons and generates an electric current.
Electrolytic Cells
Electrolytic cells, on the other hand, use electricity to drive nonspontaneous chemical reactions. In an electrolytic cell, the cathode is the negative electrode where reduction occurs, and the anode is the positive electrode where oxidation occurs. An external power source provides the electrical energy necessary to force the nonspontaneous reaction to proceed.
Key Differences
The key differences between voltaic and electrolytic cells are:
- Spontaneity: Voltaic cells generate electricity from spontaneous reactions, while electrolytic cells require external energy to drive nonspontaneous reactions.
- Direction of electron flow: In voltaic cells, electrons flow from the anode to the cathode through the external circuit. In electrolytic cells, electrons flow from the cathode to the anode through the external circuit.
- Applications: Voltaic cells are used in batteries, solar cells, and other devices that generate electricity. Electrolytic cells are used in electroplating, metal refining, and other industrial processes.
Components of Electrochemical Cells
Electrochemical cells typically consist of the following components:
- Electrodes: The cathode and anode, made of conductive materials (e.g., metals, graphite).
- Electrolyte: A solution or molten salt that conducts ions, allowing the flow of current between the electrodes.
- Separator: A porous barrier that prevents the electrodes from touching directly but allows ions to pass through.
Additional Information
- The cell potential, or voltage, of an electrochemical cell is a measure of the driving force for the reaction. In voltaic cells, the cell potential is positive, indicating a spontaneous reaction. In electrolytic cells, the cell potential is negative, indicating a nonspontaneous reaction.
- The efficiency of an electrochemical cell is determined by factors such as the electrode materials, electrolyte concentration, and temperature.
- Electrochemical cells play a crucial role in various technological applications, including energy storage, chemical synthesis, and environmental remediation.
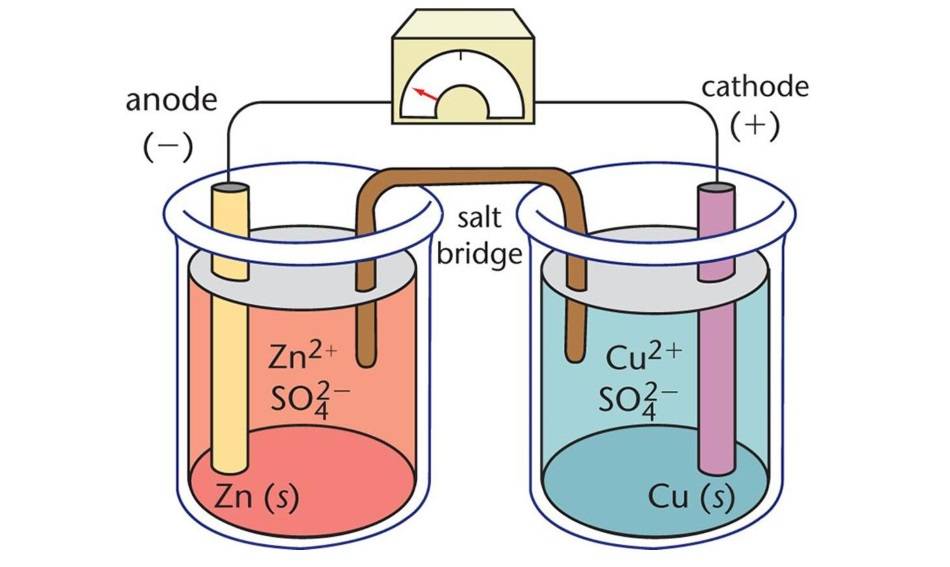
Components of Electrolytic Cells
Electrolytic cells, a type of electrochemical cell, are devices that utilize electrical energy to drive non-spontaneous chemical reactions. They consist of three primary components: the anode, the cathode, and the electrolyte.
Anode
The anode serves as the negative electrode in an electrolytic cell. During the electrochemical reaction, the anode releases electrons to the external circuit and undergoes oxidation. In other words, the anode is where the loss of electrons and oxidation occur.
Cathode
The cathode, on the other hand, acts as the positive electrode. It acquires electrons from the external circuit and is reduced during the electrochemical reaction. Thus, the cathode is where the gain of electrons and reduction take place.
Electrolyte
The electrolyte is a substance that contains mobile ions when dissolved in a solvent or melted. In electrolytic cells, the electrolyte provides a medium for the flow of ions between the anode and cathode, completing the electrical circuit. Common electrolytes include aqueous solutions of salts and molten salts.
Key Differences from Galvanic Cells
Electrolytic cells differ from galvanic cells in several key aspects:
- Cell Reactions: Electrolytic cells require an external source of electrical energy to drive non-spontaneous reactions, while galvanic cells generate electrical energy from spontaneous reactions.
- Energy Flow: Electrolytic cells consume electrical energy to promote non-spontaneous reactions, whereas galvanic cells convert chemical energy into electrical energy.
- Electrode Charges: In electrolytic cells, the anode is negative and the cathode is positive, while in galvanic cells, the anode is positive and the cathode is negative.
Working of an Electrolytic Cell
The operation of an electrolytic cell involves the following steps:
- An external power source (e.g., a battery) is connected to the electrodes of the electrolytic cell.
- The power source provides electrical energy, which drives the non-spontaneous reaction.
- At the anode, oxidation occurs, releasing electrons into the external circuit.
- The electrons flow through the external circuit to the cathode.
- At the cathode, reduction occurs, consuming electrons from the external circuit.
- Ions in the electrolyte migrate to maintain electrical neutrality, completing the circuit.
Applications of Electrolytic Cells
Electrolytic cells have numerous practical applications, including:
- Electroplating: Depositing a thin layer of metal on a surface for decorative or protective purposes.
- Electrolysis of Water: Producing hydrogen and oxygen from water, which is used in fuel cells and other applications.
- Production of Chemicals: Synthesizing various chemicals, such as chlorine, sodium hydroxide, and aluminum.
- Refining of Metals: Purifying metals by removing impurities through electrolysis.

Electrode Potentials and Redox Reactions
Electrochemical cells facilitate oxidation-reduction (redox) reactions. These cells come in two forms: galvanic (voltaic) cells, where spontaneous reactions occur, and electrolytic cells, where non-spontaneous reactions take place.
In electrochemical cells, the oxidation reaction takes place at the anode (negative terminal), while the reduction reaction occurs at the cathode (positive terminal). In electrolytic cells, the anode attracts anions, making it positive, while in galvanic cells, the anode is negative due to the release of electrons from the spontaneous oxidation reaction.
The direction of electron flow and the spontaneity of redox reactions are determined by the electrode potential, which is the potential difference between the electrodes. The standard hydrogen electrode (SHE) serves as the reference point, with a voltage of 0 volts.
The cell potential, which can be predicted using electrode potentials, provides an estimate of the potential measured. To calculate the cell potential, the half-cell reaction equations must be balanced and the voltage difference between the electrode potentials determined.
The cathode and anode play distinct roles in electrochemical cells:
Cathode:
- Positive sign due to electron consumption
- Reduction reaction occurs
- Electrons enter
Anode:
- Negative sign due to electron release
- Oxidation reaction occurs
- Electrons exit
By convention, the cathode is represented on the right-hand side, while the anode is on the left-hand side when denoting an electrochemical cell.
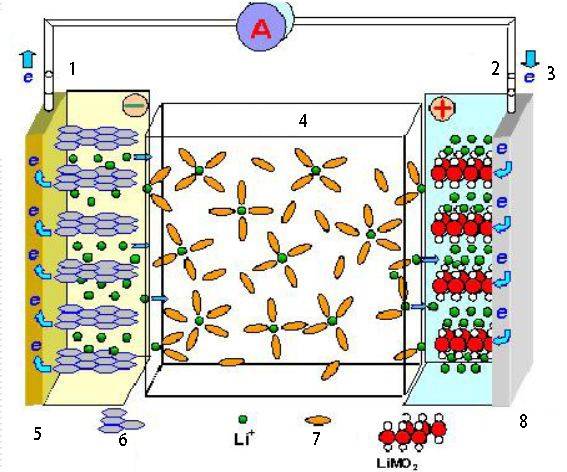
Applications of Electrochemical Cells
Electrochemical cells have a wide range of applications in various fields, including energy storage, conversion, and industrial processes.
Electroplating
Electroplating is a process that uses electrochemical cells to apply a thin layer of metal to the surface of another metal. This process enhances the properties of the base metal, such as corrosion resistance, abrasion resistance, and wear resistance. Electroplating is widely used in industries, including automotive, electronics, and jewelry, for both functional and aesthetic purposes.
Batteries
Electrochemical cells form the basis of batteries, which are essential components in numerous electronic devices and appliances. Batteries store chemical energy and convert it into electrical energy when needed. They are vital for powering portable devices, such as smartphones, laptops, and electric vehicles.
Electrowinning and Electrorefining
Electrochemical cells are employed in electrowinning and electrorefining processes to produce and purify metals. Electrowinning involves extracting metals from ores or other sources using electrolysis, while electrorefining further purifies metals by removing impurities. These processes are crucial in obtaining high-purity metals, such as copper, zinc, and aluminum, which are essential for various industrial applications.
Other Applications
Electrochemical cells also find applications in various other areas:
- Water Treatment: They are used in water electrolysis to produce hydrogen gas and oxygen gas, which are vital for fuel cells and other industrial processes.
- Fuel Cells: Electrochemical cells are the core components of fuel cells, which generate electricity through electrochemical reactions involving hydrogen and oxygen.
- Chemical Synthesis: Electrochemical cells can be utilized in chemical synthesis processes to produce various chemicals and materials.
- Sensors and Biosensors: Electrochemical cells are used in sensors and biosensors to detect and analyze specific substances based on their electrochemical properties.

Conclusion
Electrochemical cells, with their ability to convert chemical energy into electrical energy and vice versa, are indispensable tools in various fields. They serve as the foundation for energy storage, conversion, and numerous industrial processes. From batteries that power our devices to fuel cells that generate clean energy, electrochemical cells continue to shape technological advancements. Understanding their principles and applications is paramount for further innovations in energy technologies, sustainable solutions, and electrochemical engineering.
Related Products
- H Type Electrolytic Cell Triple Electrochemical Cell
- Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Side Window Optical Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- Understanding Electrolytic Cells and Their Role in Copper Purification and Electroplating
- Applications of Electrolytic Cells in Purification and Electroplating
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Overcoming Challenges with H-Type Electrolytic Cell Operation
- Applications of H-Type Electrolytic Cell in Metal Extraction




















