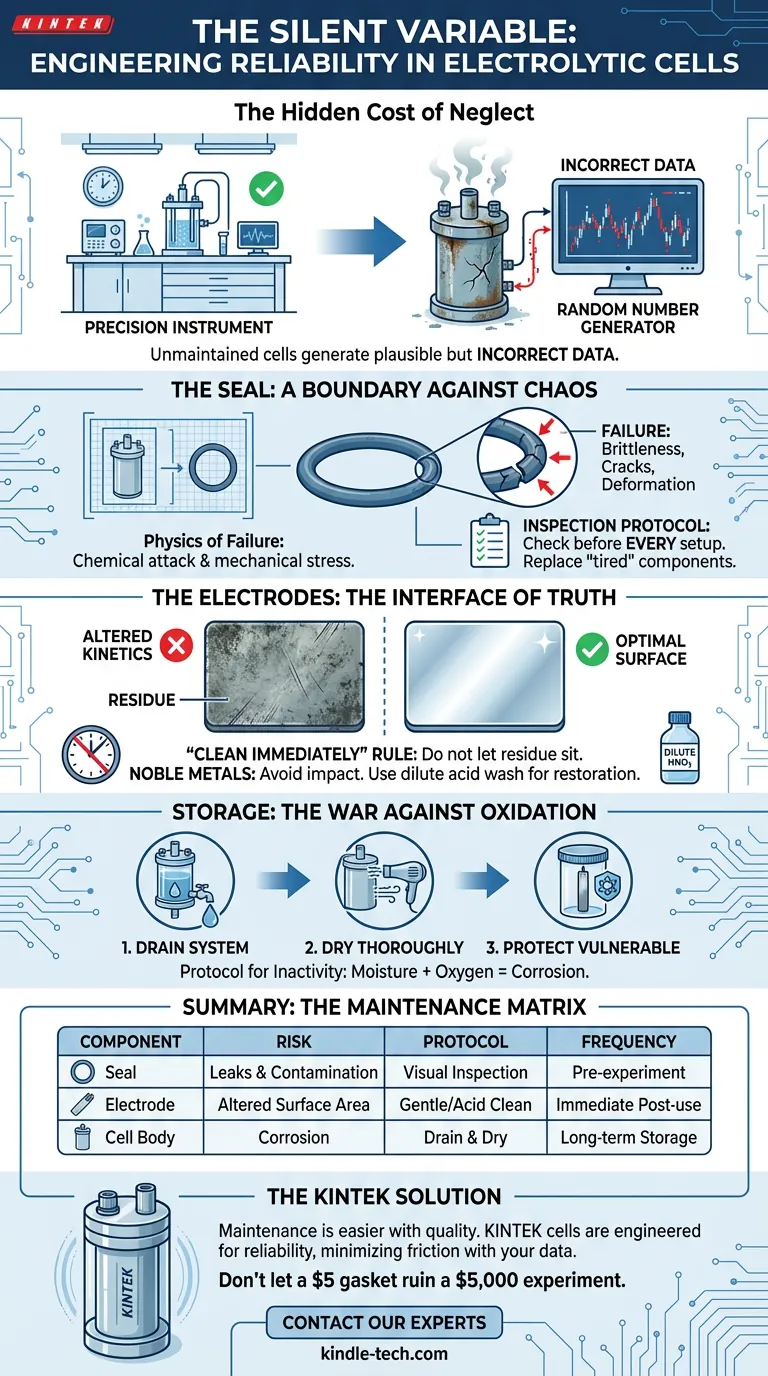
The Hidden Cost of Neglect
In the laboratory, we often obsess over the variables we can see: the purity of the reagents, the precision of the voltage, and the timing of the reaction.
We rarely think about the variables we cannot see.
Maintenance is often viewed as a chore—a tax on our time that keeps us from the "real work" of discovery. This is a psychological trap. In electrochemical experiments, the condition of your equipment is not a backdrop; it is an active participant.
An unmaintained electrolytic cell does not just stop working. It does something far worse: it begins to generate plausible but incorrect data. It becomes a random number generator disguised as a precision instrument.
Here is the engineering reality of maintaining the integrity of your cell.
The Seal: A boundary Against Chaos
The seal of an electrolytic cell is your primary defense against entropy. Its job is binary: it either maintains a closed system, or it invalidates your experiment.
There is no middle ground.
The Physics of Failure
Sealing components, primarily O-rings and gaskets, are subject to a brutal environment. They face chemical attack from electrolytes and mechanical stress from clamping.
Over time, rubber loses its elasticity. It becomes brittle. It cracks.
When a seal fails, two things happen:
- Concentration drift: Electrolyte evaporates or leaks, changing the molarity.
- Contamination: Atmospheric gases enter the system.
The Inspection Protocol
Do not wait for a leak. A leak is a lagging indicator of failure. You need leading indicators.
Before every setup, visually inspect gaskets for:
- Deformation (flattening)
- Micro-cracks
- Brittleness
If a component looks "tired," replace it. The cost of a new O-ring is negligible compared to the cost of a retracted paper.
The Electrodes: The Interface of Truth
The electrode is where the abstract concept of chemistry becomes physical reality.
The surface condition of an electrode dictates the reaction kinetics. A scratched platinum plate or a dirty carbon rod changes the effective surface area. This alters current density and catalytic efficiency.
The "Clean Immediately" Rule
Residue has a compounding interest effect. The longer reaction products sit on an electrode surface, the harder they are to remove.
The Golden Rule: Clean the electrode immediately after the experiment ends.
Use a solvent appropriate for your specific chemistry to remove surface dirt. Do not let the cell sit "until tomorrow." By tomorrow, the oxidation may be permanent.
Handling Noble Metals
Noble metals like platinum are chemically robust but physically soft.
- Avoid impact: A scratch is a canyon at the molecular level.
- Chemical restoration: For noble metals, a simple rinse often isn't enough. Soaking in dilute nitric acid (1M) followed by a deionized water rinse can reset the surface state.
Storage: The War Against Oxidation
Most equipment damage happens when the lab is dark.
How you store your electrolytic cell determines its lifespan. The greatest enemy during downtime is moisture combined with oxygen.
The Protocol for Inactivity
If you are pausing for an hour, keep it clean. If you are pausing for a week, you need a strategy.
- Drain the System: Never leave electrolyte in the cell for long-term storage. It promotes slow, corrosive reactions that eat away at seals and electrode connections.
- Dry Thoroughly: Moisture is the catalyst for oxidation.
- Protect the Vulnerable: Oxidation-prone electrodes require an oxygen-free environment or immersion in an antioxidant protective solution.
Summary: The Maintenance Matrix
Successful labs do not rely on memory; they rely on systems. Use this framework to standardize your maintenance.
| Component | The Risk | The Protocol | Frequency |
|---|---|---|---|
| Seals | Leaks & Contamination | Visual inspection for cracks/brittleness. | Pre-experiment |
| Electrodes | altered Surface Area | Gentle cleaning; Acid wash for noble metals. | Immediate Post-use |
| Cell Body | Corrosion | Remove electrolyte; store completely dry. | Long-term Storage |
The KINTEK Solution
There is a romance in precision. There is a specific satisfaction in knowing that your equipment is not a variable, but a constant.
At KINTEK, we understand that maintenance is easier when you start with quality. Our electrolytic cells and consumables are engineered to withstand the rigors of serious research, minimizing the friction between you and your data.
Don't let a $5 gasket ruin a $5,000 experiment.
Contact Our Experts to discuss your experimental setup and find the durable equipment your research deserves.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
Related Articles
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance
- The Fragility of Precision: Mastering the Integrity of Five-Port Electrolytic Cells
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell




















