The Invisible Component
In electrochemistry, we obsess over the visible variables: voltage, current, electrode material, and electrolyte concentration. We meticulously record data points to the third decimal.
Yet, the success of the entire experiment rests on an invisible component: containment.
The five-port water bath electrolytic cell is a marvel of functional geometry. It maintains thermal equilibrium while allowing complex access for electrodes and gases. But it is also a system of fragility.
When a cell leaks, it is rarely a manufacturing defect. It is almost always a failure of process. It is the result of treating the vessel as a simple bucket rather than a precision instrument.
Preventing leaks requires a shift in mindset. We must move from reacting to puddles to curating a system of perfect seals.
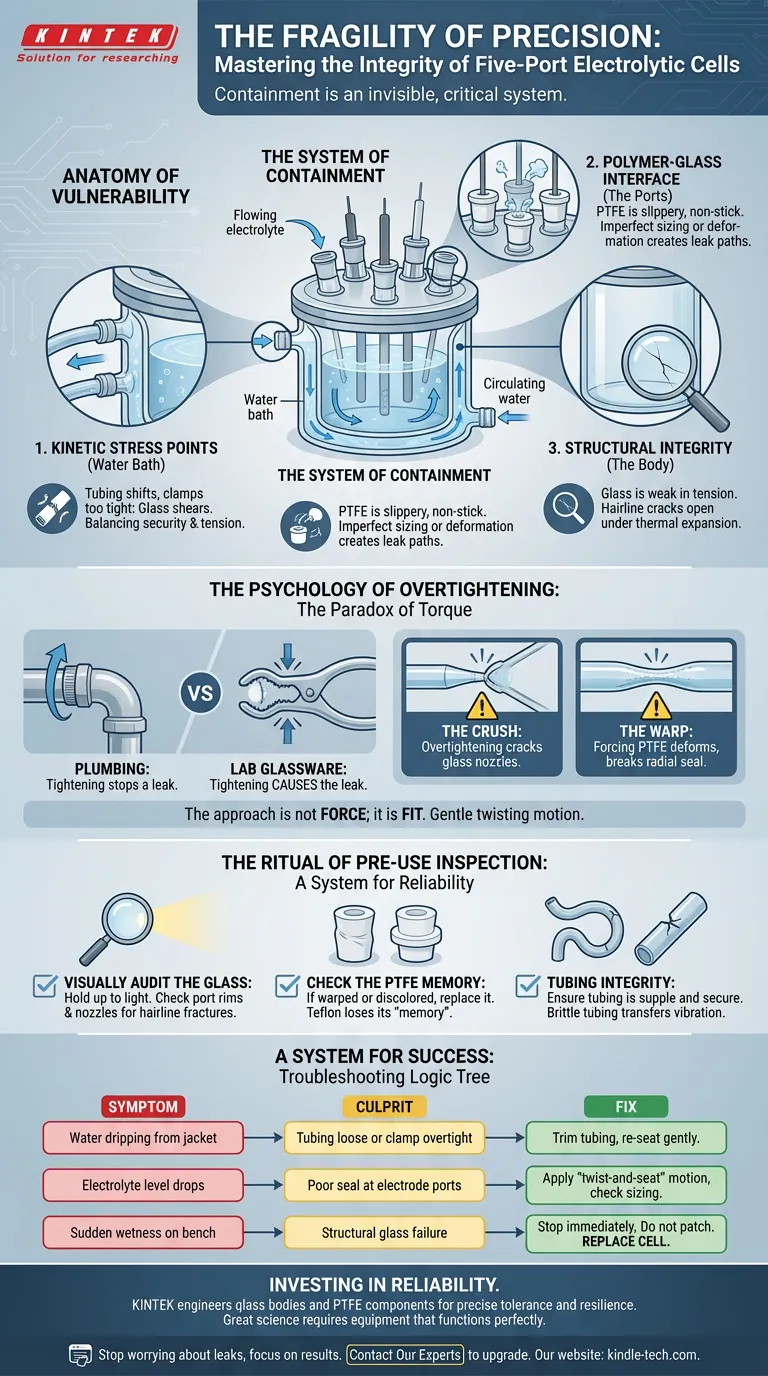
The Anatomy of Vulnerability
To protect the system, you must understand where it bleeds. A five-port cell is not a solid block; it is a collection of interfaces. Every interface is a potential exit point for your data.
1. The Kinetic Stress Points (Water Bath)
The double-walled jacket is designed for thermal control, but it endures the most mechanical abuse.
The inlet and outlet ports are tethered to circulating tubing. Every time the tubing shifts, the glass feels it. If the connection is loose, water drips on your electronics. If the clamp is too tight, the glass shears. It is a balancing act between security and tension.
2. The Polymer-Glass Interface (The Ports)
The five ports on the lid house your working, auxiliary, and reference electrodes. They are sealed with PTFE (Teflon) stoppers.
PTFE is a miracle material—chemically inert and non-stick. But it is slippery. A stopper that isn't sized perfectly, or one that has deformed over time, creates a path of least resistance for gas and liquid.
3. The Structural Integrity (The Body)
Glass is strong in compression but weak in tension. A hairline crack near a port acts like a slow-moving fault line. It may hold during setup, but once thermal expansion occurs during the experiment, the fault opens.
The Psychology of Overtightening
When we see a leak, our lizard brain takes over. We feel fear. We want to stop the flow. So, we tighten.
This is the Paradox of Torque.
In plumbing, tightening stops a leak. In laboratory glassware, tightening often causes the leak.
- The Crush: Overtightening a clamp on a water inlet cracks the glass nozzle.
- The Warp: Forcing a PTFE stopper down with brute force deforms the shape, breaking the radial seal needed against the glass wall.
The engineer’s approach is not force; it is fit. A stopper should be inserted with a gentle twisting motion, allowing the materials to mate naturally. If you have to force it, you are already breaking it.
The Ritual of Pre-Use Inspection
Reliability is not an accident. It is the result of a checklist.
Surgeons scrub in before surgery. Pilots walk around the plane before takeoff. Electrochemists must inspect their cells before the electrolyte is poured.
The Pre-Flight Checklist:
- Visually Audit the Glass: Hold the cell up to the light. Look for the glint of a hairline fracture, specifically around the port rims and the water jacket nozzles.
- Check the PTFE Memory: Teflon flows over time. If a stopper looks warped or discolored, it has lost its "memory" of the seal. Replace it.
- Tubing Integrity: Rubber becomes brittle. If the tubing connecting your water bath is stiff, it will transfer vibration to the glass. It should be supple and secure.
A System for Success
Troubleshooting a leak should not be a panic response. It should be a logic tree.
| Symptom | The Likely Culprit | The Engineering Fix |
|---|---|---|
| Water dripping from jacket | Tubing is loose or clamp is overtight. | Trim end of tubing to fresh rubber; re-seat gently. |
| Electrolyte level drops | Poor seal at electrode ports. | Apply a "twist-and-seat" motion to stoppers; check sizing. |
| Sudden wetness on bench | Structural glass failure. | Stop immediately. Do not patch. Replace the cell. |
Investing in Reliability
There is a cost to "making do" with worn equipment.
The cost is not just the replacement part. The cost is the wasted reagents. It is the lost afternoon of data. It is the safety hazard of electrified water.
At KINTEK, we view the electrolytic cell as the foundation of your research, not an accessory.
We engineer our glass bodies to withstand the thermal and mechanical rigors of daily lab use. Our PTFE components are machined for precise tolerance, ensuring that "snug" means "sealed."
Great science requires equipment that disappears into the background, functioning so perfectly that you forget it is there.
Stop worrying about leaks and start focusing on your results.
Contact Our Experts to upgrade your setup with KINTEK’s high-integrity electrolytic cells and precision consumables.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Silent Variable: Engineering Reliability in Electrolytic Cells
- The Architecture of Precision: Mastering Electrolytic Cell Maintenance
- The Architecture of Precision: Mastering the Five-Port Water Bath Electrolytic Cell
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells




















