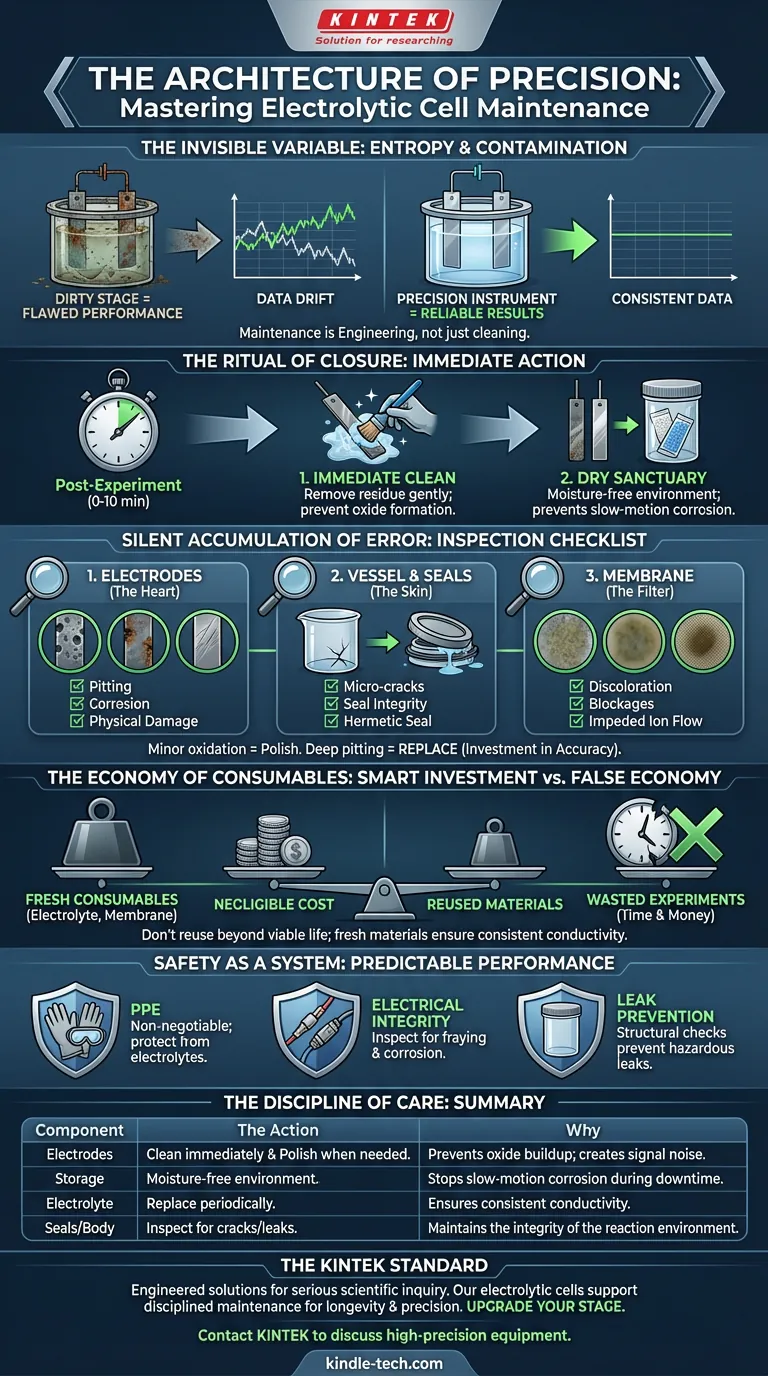
The Invisible Variable in Your Experiment
Science is often romanticized as the moment of discovery—the sudden realization or the breakthrough data point.
In reality, science is mostly cleaning.
The electrolytic cell is the theater where your electrochemical reactions perform. If the stage is dirty or the floorboards are rotting, the performance fails. Yet, maintenance is often treated as an afterthought, a chore to be rushed through so we can get back to "real work."
This is a mistake.
An unmaintained cell introduces an invisible variable into your equations: entropy. A degraded electrode or a contaminated electrolyte doesn’t just stop working; it lies to you. It produces data that looks plausible but is fundamentally flawed.
Proper maintenance isn't just hygiene. It is an engineering discipline that transforms a glass container into a precision instrument.
The Ritual of Closure
The lifespan of an electrolytic cell is rarely determined during the experiment. It is determined in the ten minutes immediately following it.
Residue is the enemy of consistency. When you leave an experiment sitting, chemical deposits begin to harden. Oxides form on the electrode surfaces. What was easily wipeable becomes a permanent scar.
The Immediate Clean
The rule is absolute: Clean immediately.
Use appropriate agents to gently remove oxides without scratching the surface. A scratch on an electrode changes its surface area, which changes your current density, which changes your results.
The Dry Sanctuary
Once cleaned, components must be dried and stored in a moisture-free environment.
Humidity is slow-motion corrosion. Storing sensitive parts, particularly electrodes, in damp conditions invites degradation that you won’t see until your baseline data starts drifting.
The Silent Accumulation of Error
Most equipment failures are not explosions. They are gradual declines.
To prevent this "data drift," a consistent inspection schedule is required. You are looking for the subtle signs of wear that signal a loss of integrity.
1. The Electrodes (The Heart)
The cathode and anode endure the most stress. Inspect them for:
- Pitting: Small craters that alter surface area.
- Corrosion: Chemical eating away of the material.
- Physical Damage: Bends or scratches.
Note on Restoration: For minor oxidation, polishing can restore performance. But understand the physics: polishing removes material. You cannot polish indefinitely. If the pitting is deep, replacement is not a cost—it is an investment in accuracy.
2. The Vessel and Seals (The Skin)
The cell body must remain hermetic. Check for micro-cracks in the glass or plastic.
More importantly, check the seals. A lid that doesn't seal tight compromises the internal environment, allowing atmosphere in or electrolyte out.
3. The Membrane (The Filter)
If your cell utilizes an ion exchange membrane, it is the most fragile component. Look for discoloration or blockages. A compromised membrane impedes ion flow, acting like a clogged artery in the system.
The Economy of Consumables
There is a psychological trap in lab management: trying to extend the life of consumables to save money.
This is false economy.
The Electrolyte: This solution is the medium of your work. Repeated experiments deplete ions and introduce contaminants. If you reuse electrolyte beyond its viable life, you are measuring the contaminants, not the reaction.
The Membrane: Do not push it past a reasonable point.
The cost of a fresh batch of electrolyte is negligible compared to the cost of a week’s worth of wasted experiments caused by bad conductive properties.
Safety as a System
Maintenance and safety are the same thing. A well-maintained machine is predictable. A neglected machine is a hazard.
- PPE: Safety goggles and gloves are non-negotiable, especially when handling electrolytes.
- Electrical Integrity: Inspect wiring for fraying. Check connections for corrosion.
- Leak Prevention: A structural check of seals prevents hazardous leaks before they happen.
Summary: The Discipline of Care
Your maintenance strategy should be a reflection of your priorities.
| Component | The Action | The "Why" |
|---|---|---|
| Electrodes | Clean immediately & Polish when needed. | Prevents oxide buildup creates signal noise. |
| Storage | Moisture-free environment. | Stops slow-motion corrosion during downtime. |
| Electrolyte | Replace periodically. | Ensures consistent conductivity. |
| Seals/Body | Inspect for cracks/leaks. | Maintains the integrity of the reaction environment. |
The KINTEK Standard
At KINTEK, we understand that a researcher is only as good as their tools.
We don't just build lab equipment; we engineer solutions designed to withstand the rigors of serious scientific inquiry. Our electrolytic cells and consumables are crafted to support a disciplined maintenance routine, ensuring longevity and precision.
When you are ready to upgrade your "stage" to a platform worthy of your work, we are here.
Contact KINTEK today to discuss how our high-precision equipment can stabilize your results and streamline your laboratory operations.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Optical Water Bath Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Fragility of Precision: Mastering the Integrity of Five-Port Electrolytic Cells
- The Silent Variable: Engineering Reliability in Electrolytic Cells




















