The most dangerous moment in any laboratory is not when an alarm sounds. It is the quiet moment right before you flip the switch.
In that second, you are relying on a chain of assumptions. You assume the glass is intact. You assume the seals are tight. You assume the electrode surface is chemically neutral.
Most of the time, these assumptions hold. But science is not about "most of the time."
In electrochemistry, entropy is patient. It hides in microscopic cracks and invisible contamination layers. A failure in a multifunctional electrolytic cell is rarely a dramatic explosion; it is usually a silent corruption of data that renders weeks of work useless.
To combat this, we do not need more luck. We need a system.
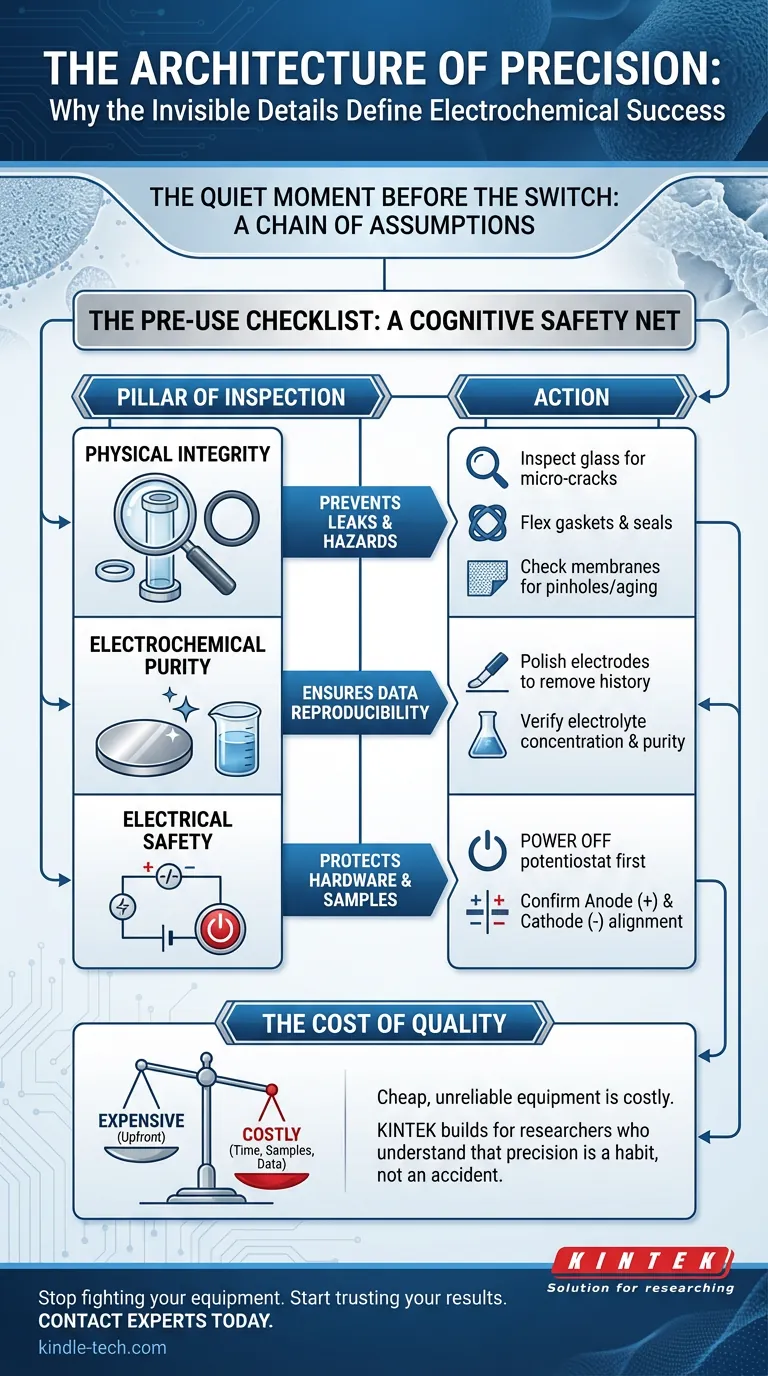
Here is the anatomy of a rigorous pre-use inspection.
The Physical Vessel: Containment is Not Optional
The cell body is the stage where the performance happens. If the stage collapses, the play is over.
Glass and specialized polymers are fragile. They endure thermal cycles and chemical stress. Over time, they develop "invisible" weaknesses.
The Visual Audit Hold the cell body up to the light. You are not just looking for obvious fractures. You are looking for:
- Micro-cracks: Hairline fractures that expand under pressure.
- Chips near ports: These compromise the seal, leading to leaks.
The Integrity of Seals Gaskets and O-rings are the unsung heroes of the cell. They must be pliable. A dry, cracked seal is a leak waiting to happen.
The Membrane Check If your cell uses an ion-exchange membrane, this is your critical failure point. A membrane allows ions to pass while separating reactants.
- Look for discoloration (aging).
- Check for pinholes (cross-contamination).
If the membrane is compromised, you aren't running an experiment; you're just mixing expensive chemicals.
The Chemical Surface: Electrodes Have Memory
The greatest lie in the lab is a "clean-looking" electrode.
An electrode surface can appear mirror-bright while still harboring an atomic layer of oxidation or organic residue from a previous experiment. In electrochemistry, the history of the electrode is the experiment.
Resetting the History The working, counter, and reference electrodes must be tabular rasa—blank slates.
- Corrosion: Check for pitting.
- Residue: Previous deposits alter reaction kinetics.
- Polish: Follow the specific protocol for your material to return the surface to its active state.
The Electrolyte Variable Never assume the purity of a stock solution.
Concentration dictates conductivity. Impurities dictate side reactions. If the electrolyte is wrong, the voltage readings are fiction. Precision requires verifying the solution every single time.
The Electrical Circuit: Polarity is Binary
There is no gray area in electrical connections. It is either correct, or it is destructive.
The most common error among even veteran researchers is a moment of distraction leading to reversed polarity.
The Connection Protocol
- Power Off: The potentiostat must be dead before you touch the leads.
- Anode vs. Cathode: Reversing these does not just give you negative data. It can strip the active material off your electrode or plate contaminants onto it, permanently ruining the hardware.
Double-check the leads. Then check them again.
Summary: The Pre-Use Checklist
A checklist is not a bureaucratic hurdle. It is a cognitive safety net.
| Pillar of Inspection | The "Why" | The Action |
|---|---|---|
| Physical Integrity | Prevents leaks and hazards. | Inspect glass for cracks; flex seals; check membranes for pinholes. |
| Electrochemical Purity | Ensures data reproducibility. | Polish electrodes to remove history; verify electrolyte concentration. |
| Electrical Safety | Protects hardware and samples. | Power OFF first; confirm Anode (+) and Cathode (-) alignment. |
The Cost of Quality
There is a distinct difference between "expensive" and "costly."
High-quality equipment may seem expensive upfront. But cheap, unreliable equipment is costly. It costs you time. It costs you samples. It costs you the integrity of your data.
When you perform these checks, you want equipment that passes. You want glass that withstands handling, seals that resist degradation, and electrodes designed for rigorous polishing cycles.
This is where KINTEK stands apart.
We build laboratory equipment for researchers who understand that precision is a habit, not an accident. Our electrolytic cells and consumables are engineered to support the rigorous standards of modern electrochemistry, reducing the variables so you can focus on the science.
Stop fighting your equipment and start trusting your results. Contact our experts today to upgrade your lab with solutions designed for accuracy.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells
- The Silent Variable: Engineering Reliability in Electrolytic Cells
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith




















