
Electrochemical Consumables
PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
Item Number : ELCP
Price varies based on specs and customizations
$29.90 / set
- Specification
- 10ml ~ 1000ml
- Applicable temperature range
- 0 ~ 60℃
- Material
- PTFE
Shipping:
Contact us to get shipping details Enjoy On-time Dispatch Guarantee.
Why Choose Us
Easy ordering process, quality products, and dedicated support for your business success.
Explore PTFE electrolytic cells with excellent corrosion resistance and optional sealing. With customizable specifications and reliable sealing, they are a great choice for your needs.
Technical specifications
Sealed PTFE electrolytic cell
| Specification | 10ml ~ 1000ml |
| Applicable temperature range | 0 ~ 60℃ |
| Sealed form | thread + apron |
| Material | PTFE |
| Electrolytic cell punching | Three electrode holes (6mm), two air holes (3mm), custom openings are available |
Unsealed PTFE electrolytic cell
| Specification | 10ml ~ 1000ml |
| Applicable temperature range | 0 ~ 60℃ |
| Material | PTFE |
| Electrolytic cell punching | Three electrode holes (6mm), custom openings are available |
Detail & Parts


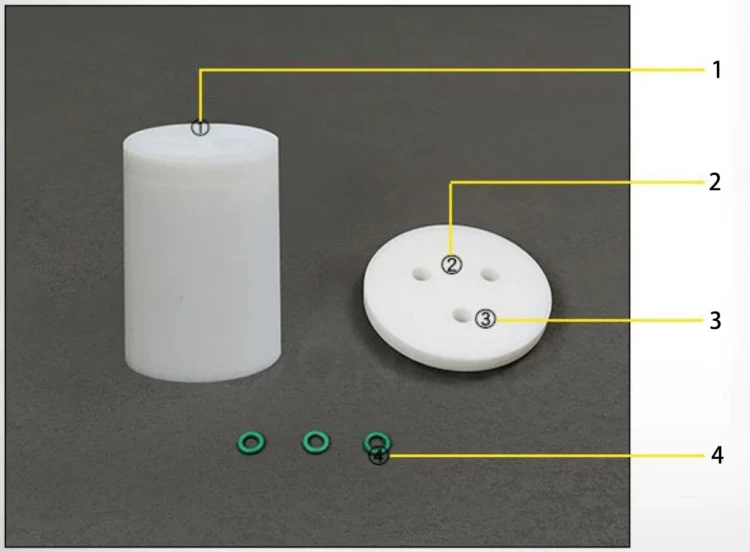





Designed for You
KinTek provide deep custom made service and equipment to worldwide customers, our specialized teamwork and rich experienced engineers are capable to undertake the custom tailoring hardware and software equipment requirements, and help our customer to build up the exclusive and personalized equipment and solution!
Would you please drop your ideas to us, our engineers are ready for you now!
Trusted by Industry Leaders

FAQ
What Are Electrolytic Cells Used For?
What Are The Materials Used In Electrochemical Cell?
What Is The Difference Between Galvanic Cell And Electrolytic Cell?
What Are The Examples Of Electrochemical Material?
What Is An Electrolytic Cell And How Does It Work?
What Are The Two Points Of Difference Between Electrochemical And Electrolytic Cells?
What Is The Example Of Electrolytic Cell?
Are Electrolytic Cells Spontaneous?
4.8 / 5
The shipment was surprisingly fast! I received it before the estimated delivery date.
4.7 / 5
As a laboratory manager, I can confidently say that this product is worth every penny. It's a fantastic investment for precise experiments.
4.9 / 5
The PTFE electrolytic cell is a game-changer. Its durability is remarkable, and it has withstood various experiments without any signs of wear.
4.6 / 5
This product is a testament to technological advancement. The design is well-thought-out and meets all our laboratory requirements.
4.8 / 5
The PTFE electrolytic cell's corrosion resistance is exceptional. It's a reliable choice for experiments involving harsh chemicals.
4.7 / 5
I highly recommend this product. It's a well-crafted piece of equipment that has significantly improved our laboratory efficiency.
4.9 / 5
The PTFE electrolytic cell is a fantastic addition to our laboratory. Its versatility makes it suitable for various experiments.
4.8 / 5
The product's quality is exceptional. It's a sturdy and reliable piece of equipment that has exceeded our expectations.
4.7 / 5
The PTFE electrolytic cell is a valuable asset to our laboratory. It's easy to use and has helped us streamline our experiments.
4.9 / 5
The product's durability is impressive. It has withstood rigorous use in our laboratory and continues to perform admirably.
4.8 / 5
The PTFE electrolytic cell is a cost-effective solution for our laboratory. It provides excellent value for money.
4.7 / 5
The product's technological advancement is commendable. It incorporates innovative features that enhance its functionality.
REQUEST A QUOTE
Our professional team will reply to you within one business day. Please feel free to contact us!
Related Products

Electrolytic Electrochemical Cell for Coating Evaluation
Looking for corrosion-resistant coating evaluation electrolytic cells for electrochemical experiments? Our cells boast complete specifications, good sealing, high-quality materials, safety, and durability. Plus, they're easily customizable to meet your needs.

Electrolytic Electrochemical Cell Gas Diffusion Liquid Flow Reaction Cell
Looking for a high-quality gas diffusion electrolysis cell? Our liquid flow reaction cell boasts exceptional corrosion resistance and complete specifications, with customizable options available to suit your needs. Contact us today!

Electrolytic Electrochemical Cell with Five-Port
Streamline your laboratory consumables with Kintek's Electrolytic Cell with five-port design. Choose from sealed and non-sealed options with customizable electrodes. Order now.

Flat Corrosion Electrolytic Electrochemical Cell
Discover our flat corrosion electrolytic cell for electrochemical experiments. With exceptional corrosion resistance and complete specifications, our cell guarantees optimal performance. Our high-quality materials and good sealing ensure a safe and durable product, and customization options are available.

Optical Water Bath Electrolytic Electrochemical Cell
Upgrade your electrolytic experiments with our Optical Water Bath. With controllable temperature and excellent corrosion resistance, it's customizable for your specific needs. Discover our complete specifications today.

Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Looking for a reliable quartz electrochemical cell? Our product boasts excellent corrosion resistance and complete specifications. With high-quality materials and good sealing, it's both safe and durable. Customize to meet your needs.

Multifunctional Electrolytic Electrochemical Cell Water Bath Single Layer Double Layer
Discover our high-quality Multifunctional Electrolytic Cell Water Baths. Choose from single or double-layer options with superior corrosion resistance. Available in 30ml to 1000ml sizes.

Button Battery Tablet Press Sealing Mold for Lab Use
The sealing die is essential for assembling button batteries, ensuring components like the anode, cathode, and electrolyte are securely enclosed.

Rotating Platinum Disk Electrode for Electrochemical Applications
Upgrade your electrochemical experiments with our Platinum Disc Electrode. High-quality and reliable for accurate results.

Proton Exchange Membrane for Batteries Lab Applications
Thin proton exchange membrane with low resistivity; high proton conductivity; low hydrogen permeation current density; long life; suitable for electrolyte separators in hydrogen fuel cells and electrochemical sensors.

No Demolding Lab Infrared Press Mold for Laboratory Applications
Effortlessly test your samples with no demolding required using our lab infrared press mold. Enjoy high transmittance and customizable sizes for your convenience.
Related Articles

The key role of PTFE in semiconductor manufacturing: from gas pipelines to electrical insulation
From high-purity gas delivery pipelines to precision electrical insulation components, the multi-faceted application of PTFE in the semiconductor industry chain provides important guarantees for the purity, stability and reliability of the manufacturing process.

Innovative Application of PTFE in Mechanical Seals
PTFE has become one of the core materials in the field of mechanical seals due to its unique chemical stability, low friction coefficient (0.04-0.15), wide temperature range (-268°C to +315°C) and excellent corrosion resistance (pH 0-14).

The Silent Dialogue: Mastering Control in Electrolytic Cells
Electrolysis is a non-spontaneous act requiring precise control. Learn to interpret the interplay of voltage, current, and physical phenomena for safer lab results.

The Vessel of Truth: Why the Container Matters More Than the Chemistry
The success of an electrolytic experiment often hangs on the material of the cell body. Discover the trade-offs between Borosilicate, Quartz, and PTFE.

Polytetrafluoroethylene (PTFE): How low friction coefficient promotes industrial progress
Explore the unique advantages of polytetrafluoroethylene (PTFE)'s low coefficient of friction and analyze how it promotes progress and innovation in industrial technology in terms of reducing wear and improving equipment efficiency.

PTFE's high temperature and corrosion resistance: Why it is indispensable in industry
The unique advantages of polytetrafluoroethylene (PTFE) in high temperature and corrosion resistance analyze why it has become an indispensable material in industry, especially in applications in harsh environments.

The Silent Variable: Engineering Reliability in Electrolytic Cells
Data accuracy depends on equipment integrity. Learn the engineering protocols for maintaining electrolytic cells to prevent systemic error.

The Unseen Variable: Mastering the Electrolytic Cell Inspection
Precision in electrochemistry begins before the current flows. Discover the critical pre-use checks for electrolytic cells that ensure safety and data integrity.

The Art of the Non-Spontaneous: Precision in Electrolytic Circuits
Mastering the electrolytic cell setup requires more than connecting wires. It demands a systematic approach to polarity, purity, and power control.

The Art of Resistance: Why Your Electrolytic Cell Needs Breathing Room
Short circuits in electrolytic cells aren't just accidents; they are geometry failures. Learn how to control the electrical path and protect your lab equipment.

The Architecture of Invisible Containment: Why Material Choice Defines Electrochemical Precision
Explore the strategic engineering behind High Borosilicate Glass and PTFE in electrolytic cells—balancing visibility with absolute chemical inertness.

The Architecture of Silence: Mastering the Super-Sealed Electrolytic Cell
Great electrochemical data starts before the experiment begins. Master the four critical steps of cell preparation—inspection, cleaning, installation, and loading.