The Invisible Component of Success
In experimental science, we often obsess over the variables we can see. We focus on the voltage, the molarity of the solution, and the composition of the catalyst.
But there is a silent variable that dictates the success of every electrochemical experiment: discipline.
An electrolytic cell is not merely a container. It is a precision instrument, a glass theatre where invisible electrons perform. If the stage is dirty, or the temperature fluctuates, the performance fails.
Properly handling a five-port water bath electrolytic cell is a systematic process. It requires treating the equipment with the same reverence a surgeon treats a scalpel. It is about risk mitigation, consistency, and the understanding that shortcuts are the quickest way to invalid data.
Here is how to master the system.
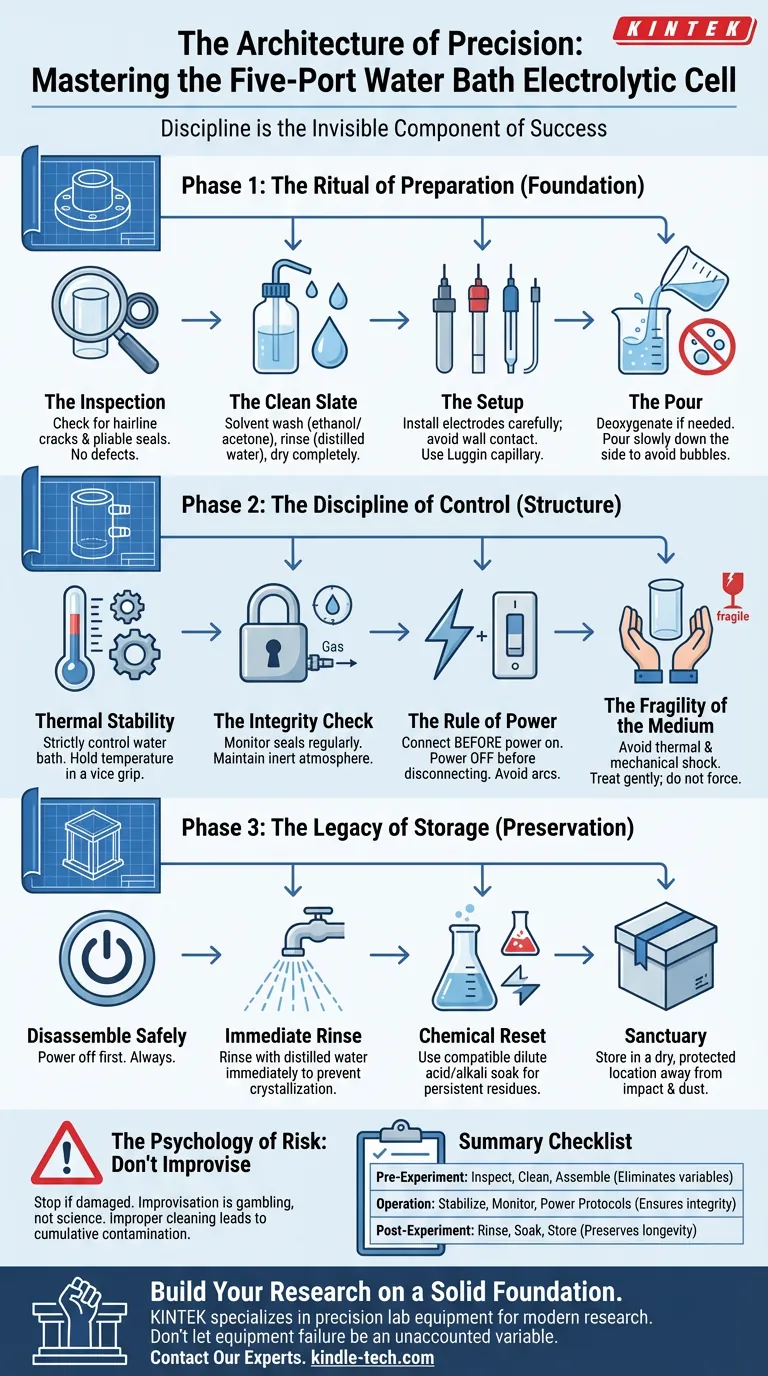
Phase 1: The Ritual of Preparation
Most experiments fail before the first drop of electrolyte is poured. They fail because of what we assume, rather than what we check.
Preparation is not a chore. It is the foundation of integrity.
The Inspection
Glass is unforgiving. Before assembly, you must examine the cell body for hairline cracks. A crack is not a cosmetic defect; it is a structural failure waiting to happen under thermal stress.
Check the seals. They must be pliable. A dry, aging seal is a leak in the making, and a leak is the end of control.
The Clean Slate
Contamination is the ghost in the machine. A fingerprint or a microscopic residue of organic matter can alter the electrochemical behavior of your system completely.
- Solvent Wash: Clean the cell body with ethanol or acetone. This removes organic residues.
- The Rinse: Follow immediately with distilled or deionized water.
- The Dry: Allow it to dry completely.
The Setup
Install the working, auxiliary, and reference electrodes with intention. They should never touch the cell wall.
If you are using a reference electrode, consider the Luggin capillary. It is a small detail that minimizes iR drop, ensuring that the voltage you measure is the voltage that exists.
The Pour
When adding the electrolyte, patience is your best tool.
If your experiment is oxygen-sensitive, perform deoxygenation first. Then, pour the solution slowly down the side of the cell. Splashing creates bubbles. Bubbles create noise. Noise ruins data.
Phase 2: The Discipline of Control
Once the experiment begins, your role shifts from architect to guardian. You are managing an environment.
Thermal Stability
Reaction kinetics are slaves to temperature. A fluctuation of a few degrees can render your kinetic data meaningless.
Strictly control the water bath circulation system. The goal is not just to reach a temperature, but to hold it in a vice grip.
The Integrity Check
Leaks are insidious. They introduce oxygen to anaerobic systems and allow dangerous gases to escape.
Regularly monitor the seals on the water bath and the cell. The cell is equipped with a gas inlet for purging; ensure this atmosphere remains inert.
The Rule of Power
Electricity commands respect. There is a simple, non-negotiable rule for electrical safety:
- Connect the cell to the potentiostat before turning the power on.
- Turn the power off before disconnecting anything.
Violating this rule risks electrical arcs. Arcs damage the delicate surfaces of your electrodes and, more importantly, can injure you.
The Fragility of the Medium
We often forget that we are working with glass in a high-temperature environment.
Avoid thermal shock. Avoid mechanical shock. Treat the cell gently. If you force a connection, you break the glass.
The Psychology of Risk
Why do we make mistakes? Usually, it is because of improvisation.
When a circulation system fails or a seal breaks, the temptation is to "make it work" to save the day's data.
Do not do this.
- The Danger of Improvisation: If a component is damaged, stop. Using a compromised cell is not science; it is gambling.
- The Cost of Contamination: Improper cleaning acts like compound interest. Residue builds up over time, eventually poisoning catalysts and creating side reactions that are impossible to separate from real data.
Phase 3: The Legacy of Storage
The experiment is not over when the data is collected. It is over when the instrument is restored.
How you leave the cell today determines how well it performs tomorrow.
- Disassemble Safely: Power off first. Always.
- Immediate Rinse: Do not let salts crystallize. Rinse the cell with distilled water immediately after discarding the electrolyte.
- Chemical Reset: If residues persist, use a dilute acid or alkali soak. But be careful—ensure the chemical is compatible with your specific cell materials.
- Sanctuary: Store the cell and electrodes in a dry, protected location. They should be safe from impact and dust.
Summary Checklist
A reliable experiment is the sum of small, correct actions.
| Phase | The Action | The Why |
|---|---|---|
| Pre-Experiment | Inspect glass, solvent clean, precise assembly. | Eliminates variables. Prevents contamination and structural failure. |
| Operation | Stabilize temperature, monitor seals, power-off protocols. | Ensures integrity. Protects the user and the validity of the data. |
| Post-Experiment | Immediate rinse, chemical soak, safe storage. | Preserves longevity. Ensures the tool is ready for the next discovery. |
Build Your Research on a Solid Foundation
You provide the discipline. The equipment provides the platform.
To get repeatable, high-quality electrochemical results, you need equipment that matches the precision of your methodology. KINTEK specializes in lab equipment and consumables designed for the rigors of modern research.
Our electrolytic cells are built to withstand the demands of thermal cycling and chemical exposure, giving you the confidence that your equipment is not the limiting factor in your research.
Don't let equipment failure be the variable you didn't account for.
Contact Our Experts regarding our electrolytic cells and let KINTEK support your pursuit of scientific truth.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- The Symphony of Coefficients: Why Your Electrolytic Cell Cannot Be a Monolith
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Fragile Vessel of Truth: A Maintenance Manifesto for Electrolytic Cells




















