Electrolysis is an act of chemical coercion.
In nature, reactions tend toward equilibrium. They want to rest. In an electrolytic cell, you are forcing a non-spontaneous reaction to occur. You are pushing a boulder up a hill using electron flow.
Because you are fighting thermodynamics, the system is unforgiving. A single misstep in the electrical architecture doesn't just pause the experiment—it reverses it, ruins it, or shatters the vessel holding it.
Great science isn't just about the hypothesis. It is about the discipline of the setup.
Here is how to engineer the perfect circuit for your electrolytic cell.
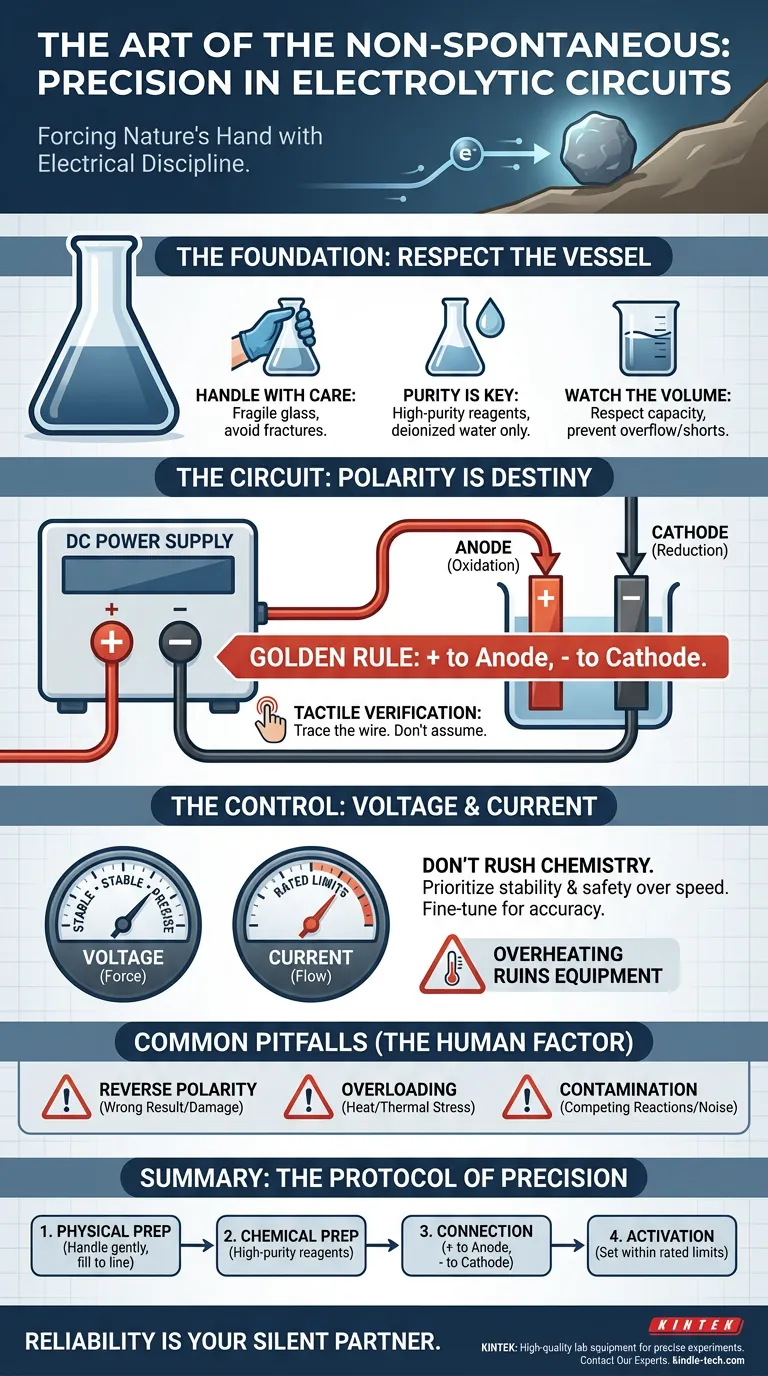
The Foundation: Respect the Vessel
Before you touch a wire, you must acknowledge the physical reality of the lab.
Most electrolytic cells are made of glass. They are the fragile stage upon which this high-energy performance takes place. The most sophisticated power supply in the world cannot compensate for a cracked beaker or a contaminated solution.
The Pre-Circuit Checklist:
- Handling: Treat the cell components with the reverence due to fragile instruments. A stress fracture now is a catastrophic leak later.
- Purity: The quality of your electrolyte defines the "noise" in your data. Use high-purity reagents and deionized water. Impurities are not just dirt; they are competing reactants that steal current and skew results.
- Volume: Pour the electrolyte carefully. Respect the maximum capacity line. Overflowing conductive liquid near electrical connections is a recipe for a short circuit.
The Circuit: Polarity is Destiny
Once the vessel is prepared, the electrical connection begins. This is where the engineer’s logic must prevail.
The defining characteristic of an electrolytic cell is that the anode and cathode have distinct, non-negotiable roles. The anode oxidizes. The cathode reduces.
If you reverse the inputs, you do not simply get "no result." You get the wrong result. You might degrade an expensive electrode or generate a gas you didn't plan for.
The Golden Rule of Connection:
- Identify the Positive (+) terminal on your DC power supply. Connect it to the Anode.
- Identify the Negative (-) terminal on your DC power supply. Connect it to the Cathode.
Do not assume. Trace the wire from the supply to the cell with your finger. This tactile verification is the difference between a technician and a master.
The Control: Voltage and Current
Connecting the wires is the anatomy; setting the power is the physiology.
The electrochemical reaction is governed by the flow of electrons (current) and the force driving them (voltage).
The error most people make is impatience. They crank the dials to "make it go faster."
But chemistry has a speed limit.
- If you prioritize safety: Never exceed the rated current/voltage of your cell. Overheating leads to thermal stress, which breaks glass and ruins equipment.
- If you prioritize accuracy: Focus on stability. A fluctuating power supply yields fluctuating chemical deposition.
- If you prioritize control: Use the fine-tune adjustments. The rate of reaction is directly proportional to your settings.
Common Pitfalls (The Human Factor)
In complex systems, errors rarely happen because of ignorance. They happen because of complacency.
We forget to check the ratings. We grab the closest reagent rather than the purest one. We assume the red wire is always positive without looking at the terminal.
Avoid these three specific failures:
- Reverse Polarity: Always double-check connections before flipping the switch.
- Overloading: Exceeding electrical limits creates heat, and heat is the enemy of precision.
- Contamination: A dirty electrolyte renders the electrical precision irrelevant.
Summary: The Protocol of Precision
Success in the lab is reproducible. It follows a rhythm.
| Phase | Action | The "Why" |
|---|---|---|
| 1. Physical Prep | Handle glass gently; fill to line. | Prevents physical breakage and dangerous spills. |
| 2. Chemical Prep | Use high-purity reagents. | Ensures the current drives the intended reaction. |
| 3. Connection | Positive (+) to Anode; Negative (-) to Cathode. | Establishes the correct direction of electron flow. |
| 4. Activation | Set voltage/current within rated limits. | Prevents overheating and ensures equipment longevity. |
The Role of Reliability
You provide the discipline. The equipment provides the reliability.
When you are trying to force nature to do the impossible, you cannot afford to worry about the quality of your tools. A power supply that drifts or a cell that leaks introduces variables that destroy the scientific method.
KINTEK understands this engineer's romance. We specialize in the high-quality lab equipment and consumables—from robust power supplies to premium electrochemical cells—that serve as the silent, reliable partner in your experiments.
When precision is the only option, Contact Our Experts to equip your lab with the standard it deserves.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Super Sealed Electrolytic Electrochemical Cell
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- H Type Electrolytic Cell Triple Electrochemical Cell
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Art of the Empty Vessel: Preparing Quartz Electrolytic Cells for Absolute Precision
- The Vessel of Truth: Why the Container Matters More Than the Chemistry
- The Invisible Variable: Why Post-Experiment Rituals Define Scientific Truth
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages




















