In experimental science, we often obsess over the variables we introduce: the voltage, the catalyst, the temperature.
We rarely give enough thought to the variables we don't intend to introduce.
A fingerprint on an electrode. A microscopic fracture in the quartz. A stray molecule of oxygen dissolved in the solution.
These are the silent killers of data. They turn a deterministic experiment into a probabilistic mess.
Proper preparation of an all-quartz electrolytic cell is not merely a chore before the "real work" begins. It is the foundation of the work itself. As surgeon Atul Gawande might argue regarding the operating room, success is rarely defined by a single stroke of genius, but by the relentless elimination of failure points before the procedure even starts.
Here is how to engineer certainty into your electrochemical setup.
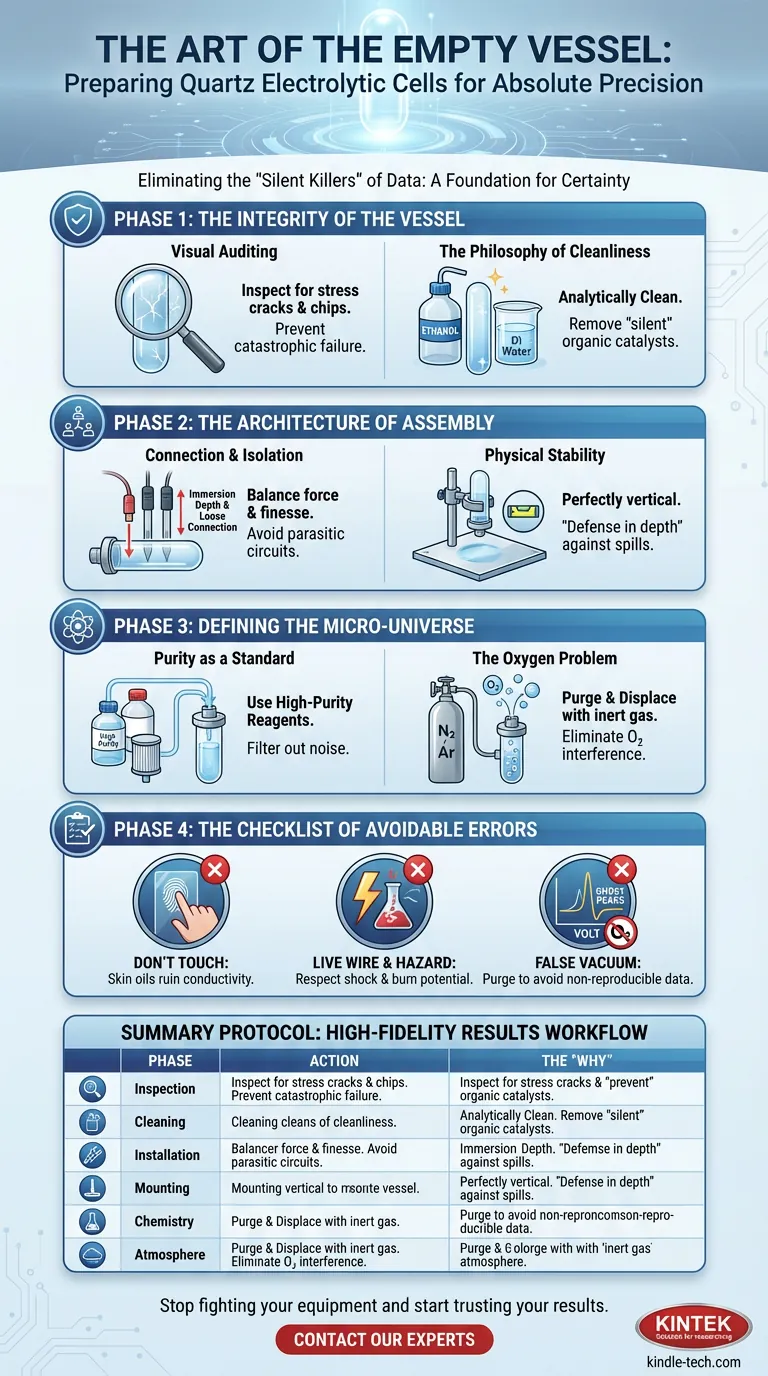
The Integrity of the Vessel
Quartz is a material of engineer’s romance. It offers optical clarity, thermal resistance, and chemical inertness. But it is brittle, and it holds grudges against mechanical stress.
Before you consider the chemistry, you must validate the physics of your container.
Visual Auditing
Start with a meticulous inspection. You are looking for stress cracks, chips, or fractures, particularly around the joints and electrode ports.
A compromised cell is a ticking time bomb. Under thermal fluctuation or mechanical load, a hairline fracture becomes a leak. A leak becomes a catastrophic failure.
The Philosophy of Cleanliness
"Clean" is a relative term. In your kitchen, a washed plate is clean. In electrochemistry, that same plate is a disaster zone of organic residue.
You need the surface to be analytically clean.
- The Solvent: Wash with high-purity ethanol to dissolve organic oils.
- The Rinse: Follow with multiple rinses of deionized (DI) water.
- The Goal: You are removing unseen catalysts. If a residue remains, it becomes a participant in your redox reaction.
The Architecture of Assembly
Once the vessel is sound, you must introduce the actors: the electrodes.
This stage requires a balance between force and finesse. The electrodes—working, reference, and counter—must be installed into their designated ports with geometric precision.
Connection and Isolation
The connections must be tight enough to prevent atmospheric leaks but loose enough to avoid stressing the quartz ports.
Crucially, watch the immersion depth. The active area must be submerged, but the electrolyte must never touch the upper connecting pins. If the fluid touches the connection point, you create a parasitic circuit. You are no longer measuring the chemistry; you are measuring the corrosion of a connector.
Physical Stability
Gravity is a variable you cannot control, so you must manage it.
Mount the cell on a laboratory stand. It must be perfectly vertical. If you are working with corrosive electrolytes, place a chemical-resistant pad beneath the setup. This is "defense in depth"—planning for a failure that you hope never happens.
Defining the Micro-Universe
Now, you introduce the chemistry. This is where you transition from physicist to chemist.
You are not just pouring liquid into a glass; you are creating a controlled atmosphere—a temporary universe where only specific laws of physics apply.
Purity as a Standard
Use high-purity reagents and DI water. If your water contains trace metal ions, those ions will migrate to your electrode and appear in your data.
Filter the solution to remove suspended microparticles. Noise in the solution equals noise in the signal.
The Oxygen Problem
Oxygen is the uninvited guest at every electrochemical party. It is electrochemically active and loves to interfere with reduction reactions.
If your experiment is sensitive (and most are):
- Purge: Flush the sealed cell with an inert gas like high-purity nitrogen or argon.
- Displace: Ensure the internal air is completely replaced before the experiment begins.
The Checklist of Avoidable Errors
Morgan Housel often writes that getting rich is about not being stupid. Similarly, getting good data is often about not making unforced errors.
Most failures in the lab are not due to complex theoretical gaps, but simple procedural lapses.
- The Touch of Death: Never touch the internal surfaces or active electrode areas with bare hands. Skin oils are insulating layers that ruin conductivity.
- The Live Wire: Electrochemical systems are live circuits. Respect the shock hazard and chemical burn potential.
- The False Vacuum: Failing to purge oxygen results in non-reproducible "ghost" peaks in your voltammograms.
Summary Protocol
The following table outlines the systematic workflow required for high-fidelity results.
| Phase | Action | The "Why" |
|---|---|---|
| 1. Inspection | Check for chips and stress cracks. | Prevents mechanical failure and leaks. |
| 2. Cleaning | Ethanol wash + DI water rinse. | Removes "silent" organic catalysts. |
| 3. Installation | Secure electrodes; check depth. | Prevents shorts and parasitic corrosion. |
| 4. Mounting | Vertical alignment + spill pad. | Ensures physical stability and safety. |
| 5. Chemistry | High-purity reagents + filtering. | Maximizes signal-to-noise ratio. |
| 6. Atmosphere | Inert gas purge (N₂/Ar). | Eliminates oxygen interference. |
Conclusion
The difference between a failed run and a publishable result is often found in the preparation. By treating the setup as a ritual rather than a chore, you ensure that the only surprises in your data are the scientific breakthroughs you were looking for.
At KINTEK, we understand that your equipment is the stage for your discovery. We specialize in high-purity lab equipment, from flawless quartz cells to precision electrodes, designed to eliminate hardware variables so you can focus on the science.
Stop fighting your equipment and start trusting your results. Contact Our Experts to discuss your specific experimental needs today.
Visual Guide
Related Products
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell with Five-Port
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Double-Layer Water Bath Electrolytic Electrochemical Cell
Related Articles
- The Silent Dialogue: Mastering Control in Electrolytic Cells
- Understanding Quartz Electrolytic Cells: Applications, Mechanisms, and Advantages
- The Geometry of Control: Why Millimeters Matter in Electrochemistry
- The Glass Heart of the Experiment: Precision Through Systematic Care
- The Architecture of Precision: Why the Invisible Details Define Electrochemical Success




















