In the laboratory, we obsess over variables. We meticulously calculate molarities, calibrate potentiostats, and refine theoretical models.
Yet, we often ignore the most critical variable of all: the vessel itself.
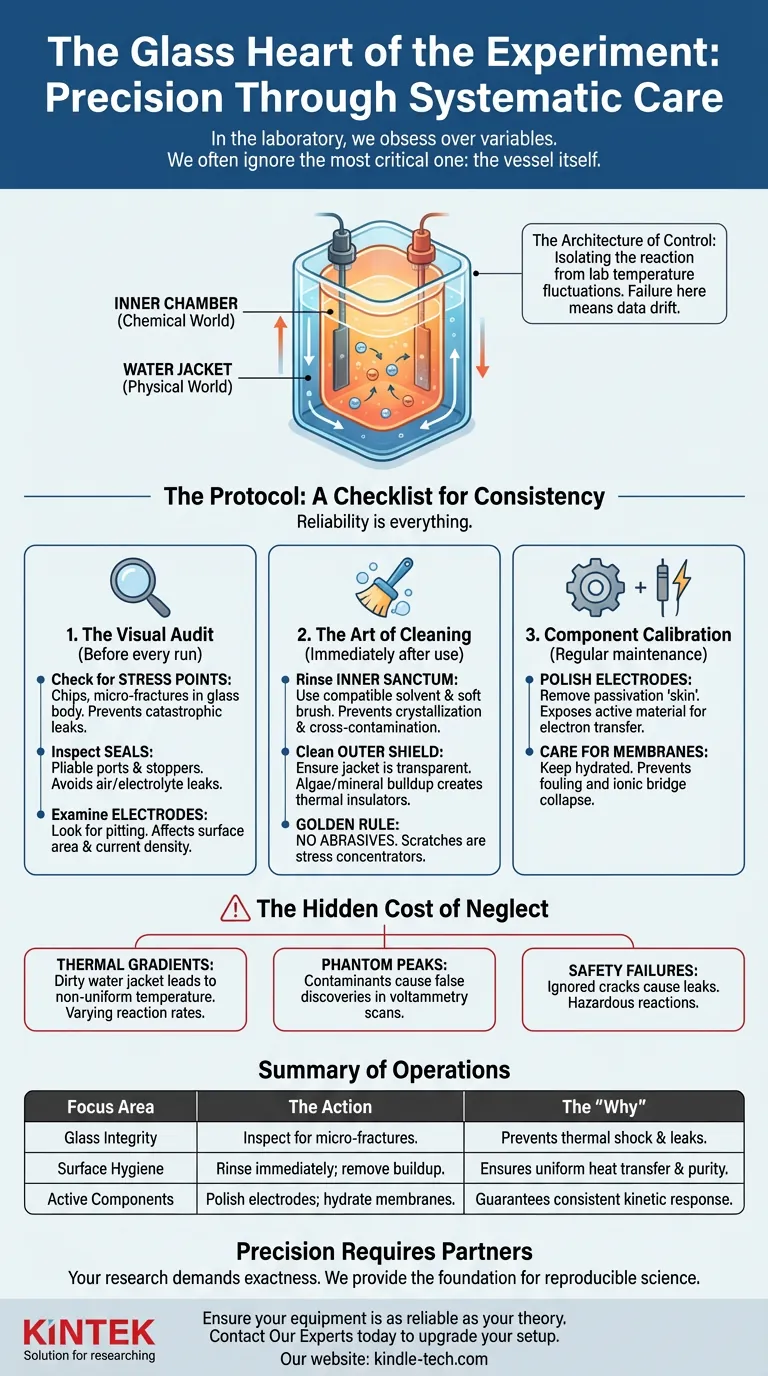
The double-layer water-bath electrolytic cell is not merely a container; it is a controlled environment. It is the stage where the invisible dance of ions occurs. When that stage is compromised—even microscopically—the data is compromised.
Reliability is boring. It doesn’t make headlines. But in electrochemistry, reliability is everything.
The difference between a breakthrough and a failed experiment is often not the science, but the systematic maintenance of the equipment that holds the science.
The Architecture of Control
To understand why maintenance matters, you must appreciate what you are asking this device to do.
A double-layer cell is an engineering solution to a thermodynamic problem. It consists of two distinct worlds:
- The Inner Chamber: This is the chemical world. It holds the electrolyte and electrodes. It is where the non-spontaneous reaction is forced into existence.
- The Water Jacket: This is the physical world. By circulating water at a specific temperature around the inner chamber, it creates a thermal shield.
The purpose of this design is isolation. It isolates your reaction from the chaotic temperature fluctuations of the lab and the heat generated by the electrolysis itself.
If the physical world (the jacket) fails to regulate the chemical world (the chamber), your reaction kinetics change. Your data drifts. You lose control.
The Protocol: A Checklist for Consistency
Complicated problems often have simple, boring solutions. The solution to experimental inconsistency is a rigorous maintenance checklist.
This is not about cleaning for aesthetics. It is about preserving the geometry and surface chemistry of your instrument.
1. The Visual Audit
Before you pour a single drop of electrolyte, look at the glass. You are looking for structural weakness.
- The Stress Points: Check the glass body for chips or fractures. Glass under thermal stress (hot inside, cold outside) is a ticking clock. A micro-crack today is a catastrophic leak tomorrow.
- The Seals: Inspect ports and stoppers. Are they pliable? Hardened seals create air leaks, introducing oxygen where you don’t want it, or allowing electrolyte to escape.
- The Electrodes: Look for pitting. A damaged electrode surface changes the active surface area. If your area calculation is wrong, your current density calculation is wrong.
2. The Art of Cleaning
Residue is the enemy of reproducibility.
If you leave electrolyte in the cell, it crystallizes. It etches the glass. It contaminates the next batch.
- The Inner Sanctum: Rinse immediately after use. Use a solvent compatible with your electrolyte. Use a soft brush.
- The Outer Shield: The water jacket must be transparent and clean. Algae or mineral buildup on the outer glass acts as an insulator. This creates "hot spots" or "cold spots," preventing uniform heat transfer.
- The Golden Rule: Never use abrasives. A scratch on the glass is a stress concentrator. You are essentially scoring the glass for a future break.
3. Component Calibration
The glass holds the fluid, but the components drive the data.
- Polishing: Electrodes passivate over time. They develop a "skin" that resists electron transfer. Regular polishing removes this layer, exposing fresh, active material.
- Membrane Care: If you use an ion-exchange membrane, treat it like a living organ. If it dries out or fouls, the ionic bridge collapses.
The Hidden Cost of Neglect
Why do we skip maintenance? Because it feels like a chore. It feels like "lost time" that could be spent running experiments.
This is a psychological trap.
When you neglect the cell, you introduce compounding errors:
- Thermal Gradients: A dirty water jacket means your solution is at 25°C near the sensor but 28°C near the wall. Reaction rates vary across the volume.
- Phantom Peaks: Contaminants from previous runs appear in your voltammetry scans, leading to false discoveries.
- Safety Failures: An ignored crack leads to a leak. Water from the jacket mixes with the organic electrolyte. In the best case, you lose the sample. In the worst case, you have a hazardous chemical reaction.
Summary of Operations
Treat your equipment with the same rigor you apply to your data analysis.
| Focus Area | The Action | The "Why" |
|---|---|---|
| Glass Integrity | Inspect for micro-fractures before every run. | Prevents thermal shock failure and leaks. |
| Surface Hygiene | Rinse immediately; remove jacket buildup. | Ensures uniform heat transfer and chemical purity. |
| Active Components | Polish electrodes; hydrate membranes. | Guarantees consistent kinetic response. |
Precision Requires Partners
Your research demands exactness. You control the chemistry, but your equipment controls the environment. There is no room for "good enough."
At KINTEK, we understand the engineer’s romance with precision. We don’t just sell lab equipment; we provide the foundation for reproducible science. From high-integrity glass cells to durable consumables, our products are designed to withstand the rigorous demands of the modern laboratory.
Don't let a dirty vessel be the reason your hypothesis fails.
Ensure your equipment is as reliable as your theory. Contact Our Experts today to upgrade your setup and maintain peak performance.
Visual Guide
Related Products
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
Related Articles
- The Unseen Variable: Mastering the Electrolytic Cell Inspection
- The Invisible Architecture of Accuracy: Optimizing the Five-Port Electrolytic Cell
- The Architecture of Control: Why Thermal Stability Defines Electrolysis Success
- Exploring the Multifunctional Electrolytic Cell Water Bath: Applications and Benefits
- The Transparency Paradox: Mastering the Fragile Art of Electrolytic Cells




















