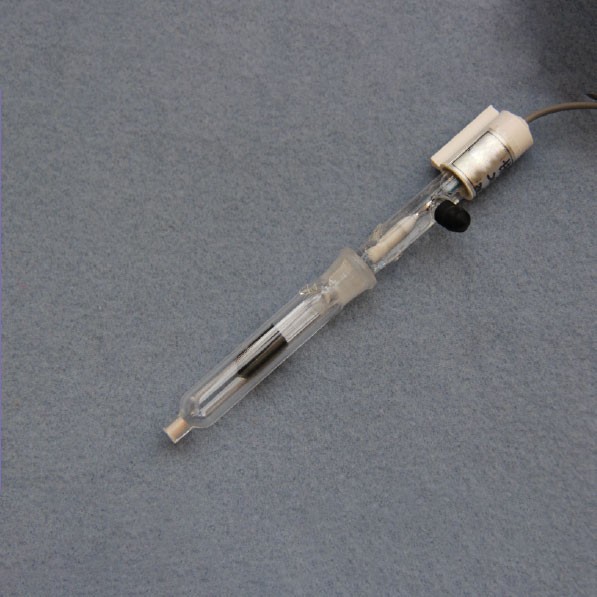
Introduction to Reference Electrodes
Reference electrodes play a crucial role in electrochemical measurements, ensuring stable and reproducible potentials. In the realm of scientific research and laboratory applications, the accuracy and reliability of these electrodes are paramount. This article delves into the specifics of saturated calomel reference electrodes (SCE), exploring their composition, operational principles, advantages, and limitations. Ideal for researchers and lab technicians, this comprehensive guide aims to enhance understanding and practical application of SCEs in various scientific contexts.
What is a Saturated Calomel Electrode (SCE)?
The Saturated Calomel Electrode (SCE) is a widely used reference electrode in electrochemical measurements, known for its stability and ease of use. It consists of a mercury (Hg) electrode coated with a layer of mercury(I) chloride, also known as calomel (Hg2Cl2), which is in contact with a saturated solution of potassium chloride (KCl). This setup ensures a consistent and reliable reference potential for various electrochemical applications.
Structure and Components
The SCE is constructed with several key components:
- Mercury (Hg): The metallic mercury acts as the electrode surface.
- Mercury(I) Chloride (Hg2Cl2): This calomel layer forms a paste with the mercury, providing the necessary chemical equilibrium for the electrode's operation.
- Saturated Potassium Chloride (KCl) Solution: The electrolyte solution is kept saturated to maintain a constant ionic activity, which in turn stabilizes the electrode potential. The saturation is crucial because it fixes the activity of the chloride ions, ensuring a stable potential.
- Platinum Wire: This component facilitates the electrical contact between the electrode and the external circuit.

Advantages and Disadvantages
Advantages:
- Ease of Setup and Reproducibility: The SCE is straightforward to prepare and can be easily reproduced, ensuring consistent results.
- Compact and Portable: Its small size and portability make it convenient for various experimental setups.
- No Separate Salt Bridge Required: The saturated KCl solution within the electrode acts as its own salt bridge, simplifying the setup.
- Stable Potential: The electrode potential remains stable over time and with minor temperature fluctuations.
Disadvantages:
- Limited Temperature Range: The SCE is typically limited to use below 50°C due to potential instability at higher temperatures.
- Interference with Certain Ions: The presence of K+ and Cl- ions in the sample can interfere with the electrochemical reactions, limiting its applicability in some scenarios.
- Potential Compensation Required: When measuring half-cell potentials, adjustments may be necessary to account for the SCE's inherent potential.
Applications
The SCE is extensively used in various fields, including analytical chemistry, environmental monitoring, and industrial processes. It serves as a reliable reference point for measuring the potentials of other electrodes, ensuring accurate and consistent data across different experiments.
In summary, the Saturated Calomel Electrode is a robust and versatile reference electrode that offers a stable potential, ease of use, and reproducibility. Despite some limitations, its advantages make it a preferred choice for many electrochemical applications.
Advantages of Using Saturated Calomel Electrodes (SCE)
Saturated Calomel Electrodes (SCE) are widely used in electrochemical experiments and applications due to their numerous advantages. These electrodes are composed of mercurous chloride (calomel) in contact with mercury metal, typically layered under a saturated solution of potassium chloride (KCl). The SCE provides a stable and reproducible reference potential, making it an essential tool in various analytical and research settings. Here, we discuss the key benefits of using SCE, including ease of setup, reproducibility, compactness, and stability of potential over time and temperature variations.
Ease of Setup
One of the primary advantages of using an SCE is its straightforward setup. The electrode is composed of simple components: calomel, mercury, and a saturated KCl solution. This simplicity reduces the complexity of assembly and minimizes the potential for errors during setup. Moreover, the SCE does not require a separate salt bridge, as it already includes a side tube containing the KCl solution. This feature simplifies the experimental setup and ensures that the electrode is ready for use with minimal preparation.
Reproducibility
Reproducibility is a critical factor in scientific research and analytical measurements. SCEs offer high reproducibility, meaning that the potential generated by the electrode remains consistent across different experiments and setups. This consistency is crucial for accurate and reliable data collection. The standardized composition and structure of SCEs contribute to their reproducibility, making them a preferred choice for researchers and analysts who require precise and repeatable results.
Compactness
SCEs are known for their compact design, which makes them convenient for use in various experimental setups. The small size of the electrode requires minimal space, allowing for more efficient use of laboratory bench space. Additionally, the compact design facilitates easy transportation, making SCEs suitable for fieldwork and on-site measurements. This portability is particularly beneficial for applications where mobility and flexibility are essential.
Stability of Potential
The stability of the potential generated by an SCE is another significant advantage. The potential of the SCE remains relatively constant over time and is minimally affected by slight changes in temperature. This stability ensures that the reference potential provided by the electrode remains accurate and reliable, even under varying experimental conditions. The consistent potential of SCEs is crucial for maintaining the integrity of electrochemical measurements and ensuring that results are valid and trustworthy.
Temperature Variations
SCEs exhibit a high degree of stability in response to temperature variations. While the electrode is typically used within a limited temperature range (up to 50°C), its potential remains relatively unaffected by small fluctuations in temperature. This temperature stability is essential for experiments and measurements that may be conducted under varying environmental conditions. The ability of SCEs to maintain a stable potential across different temperatures enhances their versatility and applicability in diverse research and analytical contexts.
In conclusion, the advantages of using Saturated Calomel Electrodes (SCE) are numerous and significant. Their ease of setup, reproducibility, compactness, and stability of potential over time and temperature variations make them an invaluable tool in electrochemical research and analysis. These benefits ensure that SCEs remain a preferred choice for scientists and analysts who require accurate, reliable, and efficient reference electrodes in their work.
Disadvantages and Limitations
The Saturated Calomel Electrode (SCE) is a widely used reference electrode in various electrochemical applications due to its stable potential and ease of preparation. However, it is not without its drawbacks and limitations. Understanding these can help in selecting the appropriate reference electrode for specific applications.
Potential Interference with Certain Ions
One of the primary limitations of the SCE is its potential interference with certain ions present in the sample. The SCE contains a saturated solution of potassium chloride (KCl), which can interfere with the measurement if the sample also contains chloride ions. This interference can lead to inaccurate readings, especially in samples with high chloride concentrations. For instance, in environmental monitoring of seawater, the high chloride content can significantly affect the accuracy of the SCE.
Need for Potential Compensation
Another limitation of the SCE is the need for potential compensation. The potential of the SCE is relatively stable, but it can vary with temperature changes. This necessitates the use of temperature compensation circuits or software in many applications to ensure accurate measurements. Without proper compensation, the potential can drift, leading to erroneous results. This is particularly important in applications where precise and accurate measurements are critical, such as in pharmaceutical and biomedical research.
Environmental and Safety Concerns Due to Mercury Content
The most significant limitation of the SCE is the environmental and safety concerns associated with its mercury content. Mercury is a toxic metal that poses serious health risks if inhaled or ingested. The use of SCEs in laboratories and industrial settings requires strict handling procedures to prevent exposure to mercury. Additionally, the disposal of SCEs and their components must comply with environmental regulations to prevent mercury contamination of water and soil.

Temperature Limitations
The SCE is also limited by its operational temperature range. The standard SCE is typically used at temperatures up to 50°C. Beyond this temperature, the stability of the electrode can be compromised, leading to potential inaccuracies in measurements. For applications requiring higher temperatures, alternative reference electrodes, such as the Silver-Silver Chloride (Ag/AgCl) electrode, are often preferred.
Chemical Compatibility
The chemical composition of the sample being measured is another critical consideration. Certain chemicals can degrade the materials used in the construction of the SCE, such as the glass or epoxy body. This can lead to a reduction in the lifespan of the electrode and potential contamination of the sample. It is essential to select the appropriate material for the electrode based on the specific application to ensure compatibility and longevity.
Conclusion
In conclusion, while the Saturated Calomel Electrode (SCE) is a reliable and widely used reference electrode, it is not without its limitations. Potential interference with certain ions, the need for potential compensation, environmental and safety concerns due to mercury content, temperature limitations, and chemical compatibility are all factors that must be considered when selecting a reference electrode for a specific application. By understanding these limitations, researchers and technicians can make informed decisions to ensure accurate and reliable measurements in their electrochemical experiments.
Applications of Saturated Calomel Electrodes
The Saturated Calomel Electrode (SCE) is a widely used reference electrode in various scientific and industrial applications due to its stable potential and ease of use. This section explores the diverse applications of SCE in different fields, including laboratory settings, environmental studies, and industrial processes, while also noting specific conditions where its use is not recommended.
Laboratory Applications
In laboratory settings, SCE is frequently used in electrochemical measurements such as pH determination, redox potential measurements, and corrosion studies. The stability of the SCE potential allows for accurate and reproducible results, making it a preferred choice for many researchers. For instance, in pH measurements, the SCE is paired with a glass electrode to determine the pH of solutions accurately. The SCE's potential remains constant, providing a reliable reference point against which the glass electrode's potential can be compared.

Environmental Studies
SCE is extensively used in environmental studies to measure the redox potential of water bodies, which is crucial for assessing water quality and the health of aquatic ecosystems. The redox potential indicates the oxidizing or reducing conditions of the water, which can influence the survival and activity of various microorganisms and the transformation of pollutants. For example, in monitoring the impact of industrial effluents on river water, SCE helps in determining the extent of oxidative stress caused by pollutants.
Industrial Processes
In industrial processes, SCE is used in corrosion monitoring and control. It is particularly useful in the oil and gas industry, where it helps in assessing the corrosion rates of metals in contact with aggressive environments. By monitoring the potential difference between the SCE and the working electrode, industries can predict and prevent corrosion, thereby extending the lifespan of equipment and reducing maintenance costs.
Limitations and Alternatives
Despite its widespread use, there are specific conditions where the use of SCE is not recommended. One major limitation is its temperature range, which is limited to 50°C. Above this temperature, the potential of the SCE becomes unstable, leading to inaccurate measurements. Additionally, the presence of certain ions, such as K+ and Cl−, can interfere with the electrochemical reactions, making SCE unsuitable for such applications.
In such cases, alternative reference electrodes, such as the Silver/Silver Chloride (Ag/AgCl) electrode, are used. The Ag/AgCl electrode is stable at higher temperatures and is less susceptible to interference from certain ions, making it a suitable choice for applications where SCE is not feasible.
Conclusion
The Saturated Calomel Electrode remains a vital tool in various scientific and industrial applications due to its stable potential and ease of use. Its applications range from laboratory measurements to environmental monitoring and industrial process control. However, understanding its limitations and knowing when to use alternative reference electrodes are crucial for obtaining accurate and reliable results. As technology advances, the development of new reference electrodes with improved performance will continue to expand the scope of applications in which these essential tools can be utilized.
Comparison with Other Reference Electrodes
When conducting electrochemical experiments, the choice of reference electrode is crucial as it provides a stable and defined potential against which other electrode potentials can be measured. Common reference electrodes include the Saturated Calomel Electrode (SCE), Silver/Silver Chloride (Ag/AgCl), Copper/Copper Sulfate (Cu/CuSO4), and the Standard Hydrogen Electrode (SHE). Each of these electrodes has its own set of advantages and disadvantages, making them suitable for different contexts.
Saturated Calomel Electrode (SCE)
The SCE is widely used due to its stability and ease of preparation. It consists of mercury in contact with a saturated solution of potassium chloride (KCl) and calomel (Hg2Cl2). The potential of the SCE is +0.241 V versus the SHE, which is a known and constant value. This makes it a reliable reference in many aqueous systems. However, its use is limited to temperatures below 50°C due to the solubility of calomel, and it is not suitable for nonaqueous systems due to the introduction of undefined junction potentials.
Silver/Silver Chloride (Ag/AgCl)
The Ag/AgCl electrode is another popular choice, especially in nonaqueous and high-temperature applications. It consists of a silver wire coated with silver chloride and immersed in a solution of KCl. The potential of the Ag/AgCl electrode varies slightly with the concentration of KCl, but it generally ranges from +0.197 V to +0.222 V versus the SHE. One of the main advantages of the Ag/AgCl electrode is its stability in a wide range of temperatures and solvents, making it versatile for various applications. However, it can be susceptible to chloride ion contamination, which affects its potential.
Copper/Copper Sulfate (Cu/CuSO4)
The Cu/CuSO4 electrode is often used in field applications due to its simplicity and robustness. It consists of a copper rod immersed in a saturated solution of copper sulfate. The potential of the Cu/CuSO4 electrode is +0.314 V versus the SHE, which is relatively stable. This electrode is particularly useful in soil and water studies where a durable and easily maintainable reference is needed. However, its potential can be affected by the purity of the copper and the concentration of the copper sulfate solution.

Standard Hydrogen Electrode (SHE)
The SHE is the primary standard for measuring electrode potentials, with a defined potential of 0.000 V. It consists of a platinum electrode in a solution with a 1 M concentration of hydrogen ions, in contact with hydrogen gas at 1 atmosphere pressure. While the SHE is the ideal reference electrode, it is impractical for routine use due to its complexity and the need for pure hydrogen gas and precise control of conditions. It is more commonly used as a theoretical reference in standard reduction potential tables.
Comparison and Contextual Suitability
Each reference electrode has its own set of advantages and limitations, making them suitable for different applications. The SCE is reliable and easy to prepare, making it a popular choice in many laboratory settings. The Ag/AgCl electrode offers versatility in both temperature and solvent, which is beneficial for nonaqueous and high-temperature applications. The Cu/CuSO4 electrode is robust and simple, ideal for fieldwork and environmental studies. The SHE, while the primary standard, is impractical for routine use but remains essential for theoretical and calibration purposes.
In summary, the choice of reference electrode should be based on the specific requirements of the experiment, including the temperature range, solvent type, and the need for stability and ease of use. By understanding the relative advantages and disadvantages of each reference electrode, researchers can make informed decisions to ensure accurate and reliable electrochemical measurements.
Selection and Considerations
When selecting a reference electrode for electrochemical measurements, several factors must be considered to ensure accurate and reliable results. The choice of reference electrode significantly impacts the quality of data obtained, and understanding the nuances of each type can help in making an informed decision.
Types of Reference Electrodes

Reference electrodes are essential components in electrochemical measurements, providing a stable and reproducible potential against which other potentials can be measured. Common types include:
- Silver/Silver Chloride (Ag/AgCl): Widely used due to its stability and relatively low cost. It is suitable for a broad range of applications, including pH measurements and general electrochemical analysis.
- Saturated Calomel Electrode (SCE): Known for its stability and ease of preparation, though it is less commonly used today due to environmental concerns associated with mercury.
- Mercury/Mercury(I) Oxide (Hg/Hg2O): Offers good stability but is less common due to similar environmental concerns as SCE.
- Mercury/Mercury Sulfate (Hg/Hg2SO4): Suitable for high-temperature applications but requires careful handling due to mercury content.
- Copper/Copper Sulfate (Cu/CuSO4): Often used in soil and groundwater monitoring due to its stability in aqueous environments.
Key Considerations
Sample Compatibility
The reference electrode must be chemically compatible with the sample to avoid any interactions that could alter the potential or react with the electrode material. For instance, certain organic solvents can dissolve some electrode materials, while aggressive ions like fluoride can attack glass or other sensitive components.
Required Potential Stability
Stability is crucial for accurate measurements. A stable reference electrode ensures that the potential remains constant over time and under varying conditions. Ag/AgCl electrodes, for example, are known for their excellent potential stability, making them a popular choice in many applications.
Response Time
The response time of a reference electrode refers to how quickly it reaches a stable potential after being immersed in the sample. Faster response times are generally preferred as they enhance the efficiency of the analytical process. Some electrodes, particularly those with porous junctions, may have slower response times due to diffusion limitations.
Temperature Considerations
Temperature can significantly affect the potential of a reference electrode. Most reference electrodes are designed for use within specific temperature ranges. For example, SCE is typically limited to 50°C. Applications requiring higher temperatures may necessitate the use of alternative electrodes, such as Hg/Hg2SO4, which can operate at higher temperatures.
Chemical Composition of Sample
The chemical composition of the sample must be considered to select an electrode that will not degrade or react with the sample components. For instance, certain chemicals can corrode the electrode body, necessitating the use of materials like glass, epoxy, or other resistant materials.
Practical Considerations
When changing the reference fill solution, it is important to note that the new potential may be less stable and more sensitive to temperature changes. Allowing the electrode to stand overnight with the new fill solution can help establish a stable potential. In practice, many users opt to purchase separate reference electrodes dedicated to specific fill solutions rather than changing the fill solution frequently.
Conclusion
Selecting the appropriate reference electrode involves a careful consideration of sample compatibility, required potential stability, response time, and temperature considerations. By understanding the strengths and weaknesses of different types of reference electrodes and their compatibility with various sample types, users can ensure accurate and reliable electrochemical measurements.
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- Platinum Auxiliary Electrode for Laboratory Use
Related Articles
- Electrochemical Electrodes in Chemical Analysis
- Reference Electrodes: Calomel, Silver Chloride, and Mercury Sulfate - A Comprehensive Guide
- A Comprehensive Guide to Reference Electrodes
- How to Make Your Own Ag/AgCl Reference Electrode for Electrochemical Experiments
- How to Choose the Right Reference Electrode for Your Application