Introduction to Reference Electrodes
Reference electrodes play a pivotal role in electrochemical measurements, serving as the stable reference point against which other electrode potentials are measured. This comprehensive guide delves into the intricacies of reference electrodes, starting with a foundational understanding of what they are and why their stability is crucial for accurate scientific research. We will explore the various types of reference electrodes, their components, and their extensive applications across different scientific domains. Whether you are a researcher or a lab technician, this guide will equip you with the knowledge to select, maintain, and troubleshoot the right reference electrode for your specific needs. Join us as we unravel the complexities of reference electrodes and their indispensable role in modern scientific investigations.
Types of Reference Electrodes
Reference electrodes play a crucial role in electrochemical measurements by providing a stable reference potential. They are essential in various applications, including pH measurement, corrosion studies, and battery performance evaluation. Reference electrodes can be broadly classified into several types: aqueous, calomel, non-aqueous, and custom-constructed. Each type has its unique characteristics and applications.
Aqueous Reference Electrodes
Aqueous reference electrodes are the most commonly used due to their stability and ease of preparation. They typically involve a metal and its salt in an aqueous solution. Some of the most common aqueous reference electrodes include:
-
Standard Hydrogen Electrode (SHE): The SHE is the universal reference electrode, defining a potential of 0.000 V. It consists of a platinum electrode in contact with hydrogen gas at 1 atmosphere pressure and an aqueous solution with a hydrogen ion activity of 1. However, the SHE is impractical for routine use due to its complexity and sensitivity to environmental conditions.
-
Saturated Calomel Electrode (SCE): The SCE is a widely used reference electrode in laboratory settings. It consists of mercury in contact with a saturated solution of potassium chloride (KCl) and mercurous chloride (calomel). The potential of the SCE is 0.241 V versus SHE at 25°C. The SCE is stable and easy to prepare, making it a popular choice for many electrochemical experiments.
-
Silver Chloride Electrode (Ag/AgCl): The Ag/AgCl electrode is another commonly used reference electrode. It consists of a silver wire coated with silver chloride and immersed in a chloride solution. The potential of the Ag/AgCl electrode is 0.197 V versus SHE at 25°C. It is highly stable and resistant to poisoning, making it suitable for a wide range of applications.
Calomel Reference Electrodes
Calomel reference electrodes are a specific type of aqueous reference electrode that use mercury and mercurous chloride. The most common calomel electrode is the SCE, as described above. Calomel electrodes are known for their stability and reliability, making them a preferred choice in many electrochemical studies.
Non-Aqueous Reference Electrodes
Non-aqueous reference electrodes are used in environments where water is not suitable, such as in organic solvents or high-temperature applications. These electrodes typically involve a metal and its salt in a non-aqueous solvent. Examples include:
-
Silver/Silver Chloride in Non-Aqueous Solvents: The Ag/AgCl electrode can be adapted for use in non-aqueous solvents by replacing the aqueous chloride solution with a non-aqueous one. This type of electrode is useful in organic electrochemistry and high-temperature applications.
-
Mercury/Mercury(I) Chloride in Non-Aqueous Solvents: Similar to the SCE, this electrode can be adapted for non-aqueous solvents by replacing the aqueous KCl solution with a non-aqueous solvent. It provides a stable reference potential in environments where water is not suitable.

Custom-Constructed Reference Electrodes
Custom-constructed reference electrodes are designed for specific applications where standard electrodes may not be suitable. These electrodes can be tailored to meet the unique requirements of a particular experiment. Examples include:
-
Copper/Copper Sulfate Electrode: This electrode is often used in soil and groundwater studies due to its stability in aqueous environments with high ionic strength. It consists of a copper rod immersed in a saturated solution of copper sulfate.
-
Dynamic Hydrogen Electrode: This electrode is used in dynamic electrochemical studies where the hydrogen gas pressure is varied to simulate different conditions. It provides a flexible reference potential for complex experiments.
In conclusion, reference electrodes are essential tools in electrochemical measurements, providing a stable and reliable reference potential. The choice of reference electrode depends on the specific requirements of the experiment, including the type of electrolyte, temperature, and environmental conditions. Understanding the characteristics and applications of different types of reference electrodes is crucial for accurate and reliable electrochemical measurements.
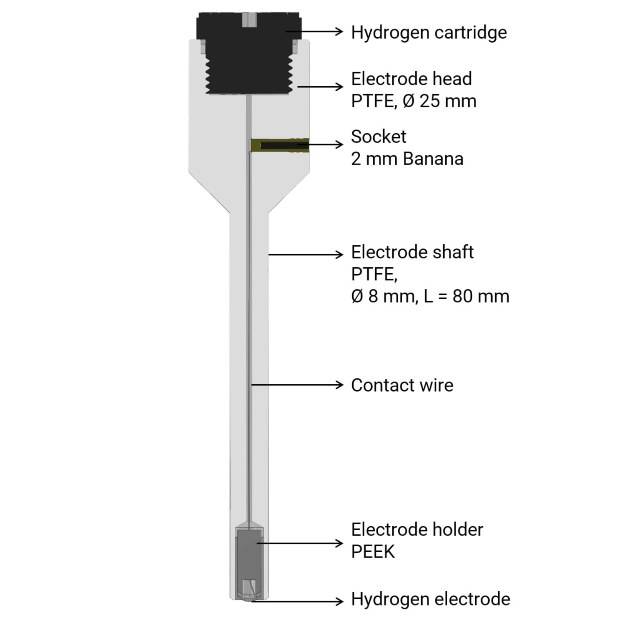
Components of Reference Electrodes
Reference electrodes are crucial components in electrochemical measurements, providing a stable and reproducible potential to which the indicating electrode potential can be compared. The key components of reference electrodes include the electrode body, internal solution, and junction. Each of these components plays a vital role in ensuring the accuracy and reliability of the electrode potential.
Electrode Body
The electrode body is the physical structure that houses the internal components of the reference electrode. It is typically made of glass or plastic, providing a durable and chemically inert container. The body must be designed to prevent any contamination of the internal solution and to ensure that the electrode remains stable under various environmental conditions. The body also includes a fill hole, which allows for the introduction of the internal solution and is sealed during storage to prevent leakage.
Internal Solution
The internal solution is a critical component of the reference electrode, providing the necessary ionic environment for the electrode to function correctly. Commonly, the internal solution consists of a saturated potassium chloride (KCl) solution, which is often saturated with silver chloride (AgCl) to enhance stability. The internal solution ensures that the reference electrode maintains a constant potential by providing a stable ionic concentration.
Junction
The junction, or liquid junction, is the point where the internal solution of the reference electrode comes into contact with the sample solution. This contact allows for the transfer of ions between the two solutions, completing the electrical circuit necessary for electrochemical measurements. The junction is typically made of a porous material, such as ceramic or glass frit, which allows for the controlled diffusion of ions while minimizing the risk of contamination.
Stability and Reproducibility
The stability and reproducibility of the reference electrode potential are influenced by several factors. The electrode body must be chemically inert and mechanically stable to prevent any changes in the electrode potential due to physical or chemical degradation. The internal solution must be carefully selected to provide a stable ionic environment, and the concentration of ions should remain constant to ensure a consistent potential. The junction must be designed to allow for controlled ion diffusion, minimizing any potential differences that could arise from uncontrolled leakage or contamination.
Non-Aqueous Reference Electrodes
In non-aqueous applications, the presence of even a small amount of electrolyte solution from the reference electrode can compromise the electrochemical reactions in the analyte solution. In such cases, pseudo-reference electrodes can be used. These electrodes, such as a platinum wire inserted directly into the analyte solution, develop a reference potential based on the solution's composition. While the potential of these pseudo-reference electrodes may change with the solution's composition, they can be calibrated using internal reference redox compounds, such as ferrocene, to ensure accurate measurements.
Construction and Maintenance
The construction of a reference electrode involves careful consideration of each component's role. The internal element, typically silver-silver chloride, must remain wet and surrounded by the reference electrolyte filling solution. This is why reference electrodes are often shipped pre-filled with the appropriate solution, and the fill hole is sealed to prevent leakage during transport. Before use, the seal must be removed to allow the fill solution to flow freely, ensuring stable and accurate readings.
The liquid junction, which needs to be kept wet for proper functioning, is often covered with a cap containing reference fill solution during storage. This helps maintain the junction's integrity and ensures that the electrode remains functional.
In conclusion, the components of reference electrodes—the electrode body, internal solution, and junction—work together to provide a stable and reproducible potential for electrochemical measurements. Understanding these components and their roles is essential for selecting the appropriate reference electrode for specific applications and ensuring accurate and reliable results.
Applications of Reference Electrodes
Reference electrodes play a crucial role in various scientific and industrial applications, particularly in electrochemistry, environmental monitoring, and biochemical analysis. These specialized electrodes provide a stable and known potential, which is essential for accurate measurements in numerous experiments and processes.
Electrochemistry
In electrochemistry, reference electrodes are used to measure the potential of other electrodes in a cell. The most common type is the standard hydrogen electrode (SHE), which is considered the universal reference with a potential of 0 V. However, due to practical limitations, other types like the saturated calomel electrode (SCE) and the silver chloride electrode (Ag/AgCl) are more frequently used in laboratory settings. These electrodes offer stability and ease of use, making them ideal for a wide range of electrochemical experiments, including corrosion studies, battery research, and fuel cell development.
Environmental Monitoring
Reference electrodes are vital in environmental monitoring, especially in the analysis of soil and water samples. They are used to measure the pH and redox potential of these samples, which are critical parameters for assessing environmental health. For instance, copper-copper sulfate electrodes are commonly used in soil testing to determine the soil's redox potential, which can influence the behavior of contaminants and the effectiveness of remediation strategies.
Biochemical Analysis
In biochemical analysis, reference electrodes are used in conjunction with other electrodes to measure the potential differences in biological systems. This is particularly important in studies involving living cells and tissues, where the accurate measurement of electrical potentials is crucial. For example, glass pH electrodes are often used in conjunction with reference electrodes to measure the pH of biological samples, which can provide insights into metabolic processes and cellular health.
Non-Aqueous Electrochemistry
Non-aqueous reference electrodes are essential in applications where the presence of water can interfere with the electrochemical reactions. In these cases, pseudo-reference electrodes, such as metal wires like platinum, are used. These electrodes develop a reference potential based on the composition of the non-aqueous solution. While they provide a stable reference potential during a single experiment, any changes in the solution composition can affect the potential. Therefore, it is common practice to add an internal reference redox compound, such as ferrocene, to ensure consistency and accuracy in the measurements.
Industrial Applications
Beyond laboratory settings, reference electrodes are used in various industrial processes. For example, they are employed in the electroplating industry to ensure uniform deposition of metals onto substrates. In the semiconductor industry, reference electrodes are used in the fabrication of microelectronic devices to control the deposition and etching processes accurately.
Conclusion
Reference electrodes are indispensable tools in scientific research and industrial applications. Their ability to provide a stable and known potential allows for precise measurements and control in a wide range of experiments and processes. Whether in electrochemistry, environmental monitoring, biochemical analysis, or industrial applications, the use of reference electrodes ensures the reliability and accuracy of the data obtained, contributing to advancements in various fields of science and technology.
Selection Criteria for Reference Electrodes
Selecting the appropriate reference electrode for a specific application is crucial for obtaining accurate and reliable electrochemical measurements. This guide will delve into the key factors to consider, including compatibility with the sample, stability, response time, temperature considerations, and the chemical composition of the sample.
Compatibility with the Sample
The reference electrode must be compatible with the sample to avoid any chemical interactions that could affect the measurement. For instance, certain chemicals can degrade the body material of the electrode. Therefore, it is essential to choose the correct material, such as glass, epoxy, or other specialized materials, to suit the application.
Stability
Stability is a critical factor in selecting a reference electrode. The electrode must provide a constant and defined potential to ensure accurate measurements. Most reference electrodes are combination electrodes, combining a stable reference and a working cell (half-cell) in one probe. However, in some applications, it may be more practical to use separate sensing and reference electrodes, especially if the different parts of the electrode are expected to have different lifespans.
Response Time
The response time of a reference electrode is another important consideration. A fast response time ensures efficiency in the analytical process. Slow or erratic response times can lead to inaccurate measurements and prolonged analysis times.
Temperature Considerations
Temperature plays a significant role in the performance of reference electrodes. For example, the saturated calomel electrode (SCE) has a limited temperature range of up to 50°C. If the application requires use at higher temperatures, an alternative electrode must be selected. It is essential to choose an electrode that can maintain stability and accuracy across the required temperature range.
Chemical Composition of the Sample
The chemical composition of the sample is a crucial factor in selecting a reference electrode. Certain chemicals can degrade the body material of the electrode, leading to inaccurate measurements and potential damage to the electrode. It is important to choose an electrode with a body material that is resistant to the specific chemicals present in the sample. Common materials include glass, epoxy, and other specialized materials designed to withstand specific chemical environments.
Available Options
A range of reference electrodes are available, each with its own advantages and limitations. Some of the most common reference systems include:
- Saturated Calomel (Hg/HgCl): This electrode is very stable but contains mercury, making it unsuitable for use in certain applications such as food, beverage, or environmental studies. Its disposal must also be carefully controlled due to environmental implications.
- Ag/AgCl (wire or cartridge): This is the most common type of reference system. It is suitable for a wide range of applications but may not be compatible with samples containing silver or chloride.
- Cu/CuSO4: This electrode is suitable for specific applications where copper sulfate is compatible with the sample.
- Hg/HgSO4: This electrode is suitable for high-temperature applications but is less common due to its mercury content.
- Hg/HgO: This electrode is suitable for high-temperature applications but is also less common due to its mercury content.
Double Junction Electrodes
Double junction electrodes have a lower chamber that contains an electrolyte that differs from the electrolyte in the top reference chamber. The chemical composition of the lower chamber electrolyte can be customized to match (or be more compatible with) the sample. This is important because the lower chamber electrolyte comes into contact with the sample via the junction, and if there is an interaction between the electrolyte and sample, it can cause the junction to block and give erratic readings.
Practical Aspects
When selecting a reference electrode, it is essential to consider practical aspects such as cost, availability, and machinability. The relative importance of these factors will vary according to the specific process. For example, in applications focused on energy or bulk-scale commodity production, small, single-digit differences in efficiency gains can be extremely critical. However, in organic synthesis where the scales are comparatively smaller, larger gains in yield or complete switches in selectivity become more important.
In conclusion, selecting the appropriate reference electrode involves a careful consideration of compatibility with the sample, stability, response time, temperature considerations, and the chemical composition of the sample. By understanding the various types of reference electrodes and their relative strengths and weaknesses, you can make an informed decision that ensures accurate and reliable electrochemical measurements for your specific application.
Maintenance and Troubleshooting
Maintaining reference electrodes is crucial for ensuring their long-term performance and accuracy in electrochemical measurements. This section provides comprehensive guidance on how to maintain reference electrodes, along with common issues and troubleshooting tips to help users address potential problems effectively.
Regular Maintenance Practices
-
Cleaning: Regular cleaning is essential to prevent contamination and ensure the electrode's longevity. Clean the electrode with distilled water and a soft brush to remove any deposits or residues. Avoid using abrasive materials that could damage the electrode surface.
-
Filling Solution Replacement: The reference electrode's filling solution should be replaced periodically to maintain its saturation and prevent the formation of crystals. Use a saturated solution of the appropriate salt (e.g., KCl for silver/silver chloride electrodes) and ensure that the solution is free from impurities.
-
Junction Maintenance: The liquid junction, often a porous frit or ceramic disc, must be kept clean and unobstructed. Regularly inspect the junction for any signs of blockage or damage. If necessary, clean the junction with a gentle stream of distilled water or replace it if it appears damaged.
-
Storage: When not in use, store reference electrodes in a solution that maintains their activity. For instance, silver/silver chloride electrodes should be stored in a saturated KCl solution. Ensure that the storage solution is fresh and free from contaminants.
Common Issues and Troubleshooting
-
Crystal Formation: Crystals at the bottom of the electrode are typically salt crystals from the filling solution. This is normal and can be managed by draining the electrode, rinsing it with distilled water to dissolve the crystals, and refilling it with fresh saturated solution.
-
Drift and Instability: If the electrode potential drifts or becomes unstable, check the filling solution's saturation and the condition of the liquid junction. Ensure that the electrode is not exposed to extreme temperatures or contaminants.
-
High Resistance: High electrical resistance can be caused by a blocked or dried-out junction. Clean or replace the junction and ensure that the filling solution is at the correct level.
-
Contamination: Contamination can occur if the electrode comes into contact with foreign substances. Regularly clean the electrode and use only distilled or deionized water for rinsing.
-
Electrode Poisoning: Certain substances can "poison" the electrode, rendering it unresponsive. Avoid exposing the electrode to heavy metals, strong oxidizing agents, or reducing agents. If poisoning is suspected, clean the electrode thoroughly or consider replacing it.

Advanced Troubleshooting
-
Potential Shift: A sudden shift in the electrode potential may indicate a change in the filling solution's composition. If you change the filling solution, allow the electrode to stabilize overnight before using it for measurements.
-
Temperature Effects: Reference electrodes are sensitive to temperature changes. Ensure that the electrode is at a stable temperature before taking measurements. Use a temperature-compensated meter if necessary.
-
Electrode Life: The lifespan of a reference electrode depends on its usage and maintenance. Regularly monitor the electrode's performance and replace it if it shows signs of degradation, such as slow response times or erratic potentials.
By following these maintenance practices and troubleshooting tips, users can ensure that their reference electrodes provide accurate and reliable measurements over time. Regular care and attention to detail are key to maintaining the integrity and performance of these essential tools in electrochemical analysis.
Comparison with Indicator Electrodes
In potentiometric analysis, the roles of reference electrodes (RE) and indicator electrodes (IE) are distinct and complementary, each serving a specific function crucial for accurate measurements. Understanding these roles is essential for anyone involved in electrochemical experiments or analytical chemistry.
Distinct Roles in Potentiometric Analysis
Reference Electrodes (RE): These are the stable and fixed electrodes in a potentiometric setup. The primary function of a reference electrode is to provide a stable and well-known potential against which the potential of the indicator electrode can be measured. This stability is crucial because it ensures that any changes in the measured potential are due to changes in the analyte and not due to fluctuations in the reference electrode's potential. Common examples of reference electrodes include the saturated calomel electrode (SCE), silver/silver chloride electrode, and the standard hydrogen electrode (SHE).
Indicator Electrodes (IE): Unlike reference electrodes, indicator electrodes are designed to respond to changes in the concentration of the analyte. They are sensitive to specific ions or substances in the solution being analyzed. The potential of an indicator electrode varies according to the activity or concentration of the analyte, making it a key component in detecting the end point of titrations or in measuring the concentration of specific ions. Examples of indicator electrodes include glass electrodes for pH measurements, metal ion indicator electrodes, and various membrane-based electrodes.
Types of Indicator Electrodes
Indicator electrodes can be categorized based on the type of membrane they use:
- Glass Membrane IE: Commonly used for pH measurements, these electrodes contain a thin glass membrane that is sensitive to hydrogen ions.
- Crystal Membrane IE: These electrodes use a single crystal or a pressed disc of a specific ion-selective material, such as lanthanum fluoride for fluoride ion measurements.
- Polymer Membrane IE: Incorporating ion-exchange materials within a polymer matrix, these electrodes are versatile and can be designed for a wide range of ion-specific applications.
Complementarity in Measurements
The effectiveness of potentiometric analysis depends on the correct pairing of reference and indicator electrodes. The reference electrode provides a stable baseline potential, while the indicator electrode responds to the analyte, allowing for precise measurements of ion concentrations or detection of titration end points. This synergy ensures that the data obtained is reliable and accurate, reflecting true changes in the sample rather than artifacts from the measurement system itself.
In summary, while reference electrodes offer stability and a known potential, indicator electrodes provide sensitivity and specificity to the analyte. Together, they form a robust system for potentiometric analysis, enabling a wide range of applications from routine laboratory tests to sophisticated research studies. Understanding the distinct roles and types of these electrodes is crucial for optimizing experimental setups and interpreting results accurately.
Future Trends in Reference Electrode Technology
The field of reference electrode technology is poised for significant advancements driven by innovative materials, improved designs, and the integration of nanotechnology. As electrochemical applications expand into new domains, including non-aqueous systems and high-precision measurements, the demand for more robust, stable, and versatile reference electrodes is increasing. This section explores the emerging trends and potential innovations that could reshape the landscape of reference electrode technology in the coming years.
Advancements in Material Science
One of the most promising areas of development in reference electrode technology is the use of advanced materials. Traditional reference electrodes often rely on materials like silver/silver chloride or calomel, which, while reliable, have limitations in terms of stability and applicability across various environments. The introduction of new materials, particularly those with nanoscale properties, offers a pathway to overcome these limitations.
Nanomaterials, due to their high surface-to-volume ratios and unique electronic properties, can significantly enhance the sensitivity and stability of reference electrodes. For instance, the incorporation of graphene or carbon nanotubes into the construction of reference electrodes can improve their conductivity and resistance to environmental interferences. Additionally, the use of metal oxides and other composite materials is being explored to enhance the durability and performance of reference electrodes in harsh conditions.
Integration of Nanotechnology
The synergy between nanotechnology and electrochemical sensing is leading to breakthroughs in reference electrode design. Nanostructures, with their diverse morphologies, are being utilized to increase the sensitivity of electrochemical measurement methods. The ability to synthesize and fabricate materials at the nanoscale, along with the control over their shape, size, arrangement, and composition, is driving the development of more efficient and precise reference electrodes.
For example, the use of nanowires and nanoparticles can provide a more uniform and stable potential across the electrode surface, reducing the variability and drift commonly associated with traditional reference electrodes. Moreover, nanotechnology enables the creation of miniaturized reference electrodes that are suitable for microfluidic and portable devices, broadening the applicability of electrochemical sensors in point-of-care testing and field applications.
Non-Aqueous Reference Electrodes
The expansion of electrochemical applications into non-aqueous systems is another trend that is shaping the future of reference electrode technology. Traditional aqueous reference electrodes can be compromised in non-aqueous environments due to the leakage of electrolyte solutions, which can interfere with the electrochemical reactions. The development of non-aqueous reference electrodes, or pseudo-reference electrodes, is therefore crucial for these applications.
Pseudo-reference electrodes, such as metal wires inserted directly into the analyte solution, offer a simpler and more adaptable solution. However, their stability and reproducibility can be challenging. Innovations in this area include the use of internal reference redox compounds with well-defined potentials, such as ferrocene, to calibrate the reference potential. This approach ensures that the reference potential remains consistent even with changes in the solution composition.
Smart and Self-Calibrating Reference Electrodes
The integration of smart technologies and self-calibrating mechanisms is another frontier in reference electrode development. Smart reference electrodes can monitor their own performance and automatically adjust to maintain optimal conditions. This includes the ability to detect and compensate for drift, contamination, and other factors that can affect the electrode's stability.
Self-calibrating reference electrodes use built-in sensors and feedback systems to continuously adjust the electrode potential, ensuring accurate and reliable measurements over extended periods. This technology is particularly beneficial for long-term monitoring applications where manual calibration is impractical or impossible.
Conclusion
The future of reference electrode technology is bright, with numerous innovations on the horizon that promise to enhance the stability, sensitivity, and versatility of these essential components in electrochemical measurements. Advancements in material science, the integration of nanotechnology, the development of non-aqueous reference electrodes, and the creation of smart and self-calibrating systems are all contributing to a new era of reference electrode technology. As these innovations continue to evolve, they will enable more accurate and reliable electrochemical measurements across a broader range of applications, driving progress in fields such as environmental monitoring, healthcare, and industrial process control.
Related Products
- Electrode Fixture for Electrochemical Experiments
- Copper Sulfate Reference Electrode for Laboratory Use
- Platinum Auxiliary Electrode for Laboratory Use
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Gold Disc Electrode



















