The rotary evaporation method of purification is a technique used to separate solvents from mixtures, particularly in organic chemistry. It involves placing a solution in a rotary evaporator, where the solvent is evaporated under reduced pressure and collected in a separate flask. The process relies on lowering the boiling point of the solvent using a vacuum, rotating the flask to increase the surface area for evaporation, and condensing the vapor back into a liquid. This method is efficient for concentrating solutions or isolating compounds without exposing them to high temperatures, making it ideal for heat-sensitive materials. Below is a detailed explanation of the key steps and principles involved in this process.
Key Points Explained:
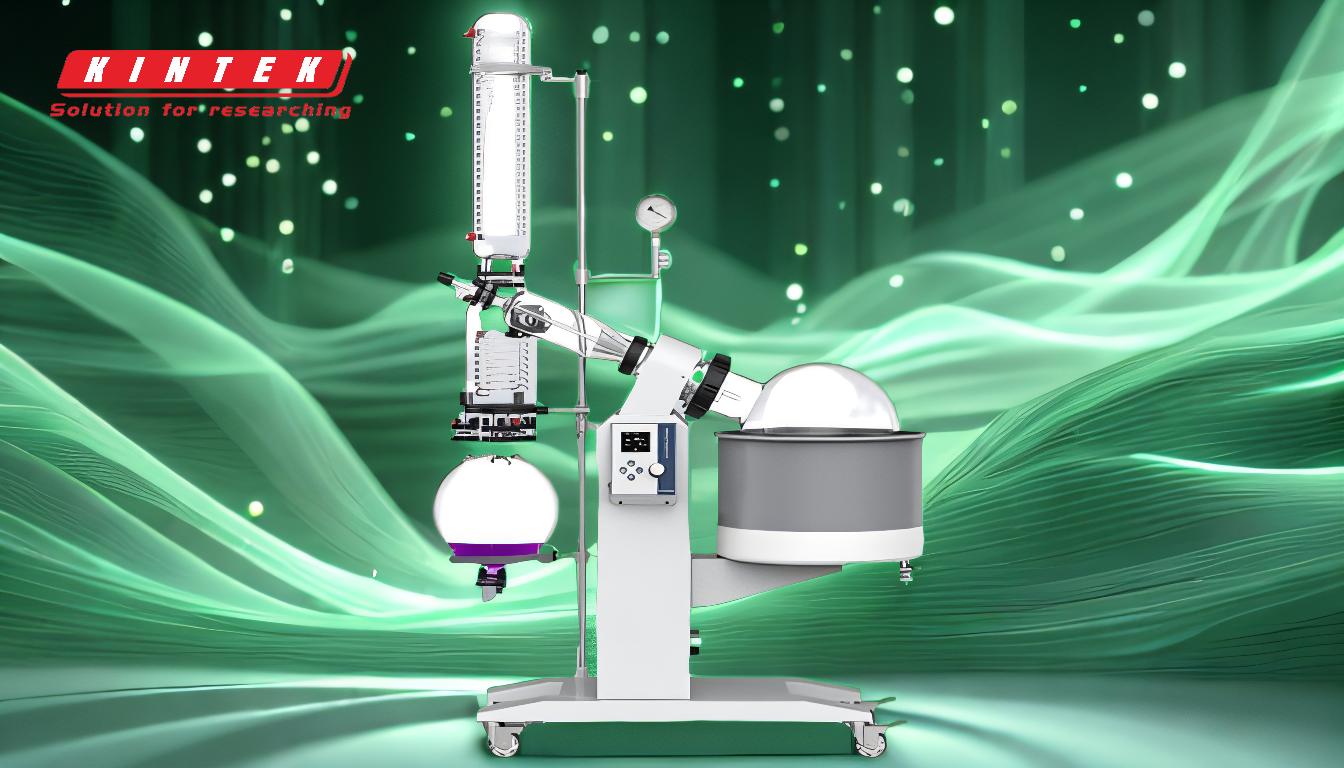
-
Setup and Preparation:
- Ensure the rotary evaporator is properly assembled, including the water bath, condenser, vacuum pump, and receiving flask.
- Check that the water condenser is functioning and that the water bath is set to the appropriate temperature.
- Secure the sample flask using clamps and attach it to the rotary evaporator, ensuring a tight seal to maintain vacuum pressure.
-
Application of Vacuum:
- Turn on the vacuum pump to reduce the pressure inside the system, which lowers the boiling point of the solvent.
- Gradually increase the vacuum to avoid sudden boiling or bumping, which can cause sample loss.
-
Rotation of the Flask:
- Start the rotation of the flask at a speed suitable for the volume of the solution (typically 150-200 rpm).
- Rotation creates a thin film of the solution on the inner surface of the flask, increasing the surface area and promoting faster evaporation.
-
Heating the Solution:
- Lower the flask into the preheated water bath, which provides controlled heating to the solution.
- The temperature of the water bath should be set below the boiling point of the solvent under reduced pressure to prevent overheating.
-
Condensation and Collection:
- As the solvent evaporates, the vapor travels through the vacuum system to the condenser.
- The condenser cools the vapor, converting it back into a liquid, which is collected in the receiving flask.
-
Monitoring and Completion:
- Continuously monitor the process to ensure the solvent is evaporating smoothly and that no bumping occurs.
- Once the solvent has been completely removed, raise the flask out of the water bath, stop the rotation, and turn off the vacuum pump.
-
System Release and Cleanup:
- Carefully open the vacuum tap to release the system from reduced pressure.
- Remove the sample flask and the receiving flask, ensuring proper handling of the purified compound and collected solvent.
- Clean and prepare the rotary evaporator for the next use.
-
Key Principles:
- Reduced Pressure: Lowering the pressure reduces the boiling point of the solvent, allowing evaporation at lower temperatures.
- Increased Surface Area: Rotation of the flask creates a thin film, enhancing evaporation efficiency.
- Temperature Control: The water bath provides controlled heating, while the condenser ensures efficient cooling and collection of the solvent.
By following these steps and principles, the rotary evaporation method effectively purifies or concentrates solutions, making it a valuable tool in laboratories for handling heat-sensitive or volatile compounds.
Summary Table:
| Step | Key Actions |
|---|---|
| Setup and Preparation | Assemble rotary evaporator, check condenser, secure sample flask. |
| Application of Vacuum | Turn on vacuum pump, gradually increase pressure to avoid bumping. |
| Rotation of the Flask | Rotate flask at 150-200 rpm to create a thin film for faster evaporation. |
| Heating the Solution | Lower flask into preheated water bath below solvent's boiling point. |
| Condensation and Collection | Cool vapor in condenser, collect solvent in receiving flask. |
| Monitoring and Completion | Monitor process, stop rotation, and turn off vacuum pump when complete. |
| System Release and Cleanup | Release vacuum, handle purified compound, and clean equipment. |
| Key Principles | Reduced pressure, increased surface area, and temperature control. |
Discover how rotary evaporation can enhance your lab’s efficiency—contact our experts today!










