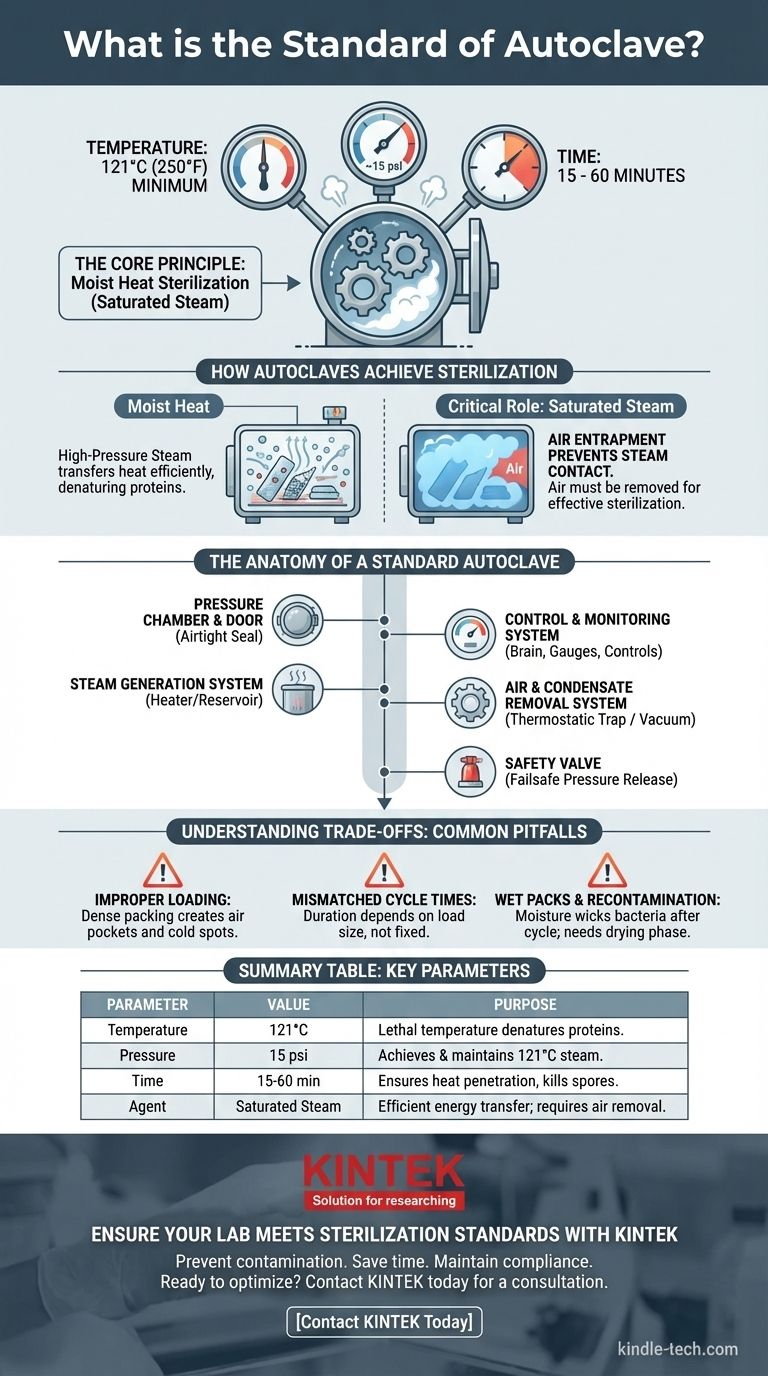
To be effective, a standard autoclave cycle operates at a minimum temperature of 121°C (250°F) and a pressure of approximately 15 psi for a duration of 15 to 60 minutes. These parameters are not arbitrary; they are the precise conditions required for saturated steam to penetrate and destroy all forms of microbial life, including heat-resistant spores.
The "standard" for an autoclave is not just a set of numbers, but a principle: using pressurized, saturated steam to achieve a lethal temperature. Understanding how the components work together to achieve this principle is the key to ensuring true sterilization, not just heating.

The Core Principle: How Autoclaves Achieve Sterilization
An autoclave's effectiveness is rooted in a fundamental law of physics. It operates on the principle of moist heat sterilization, which is significantly more effective than dry heat.
It's All About Moist Heat
High-pressure steam is the active agent in an autoclave. Pressure is used to increase the boiling point of water far beyond 100°C.
This superheated steam can transfer heat energy to items much more efficiently than dry air, leading to a rapid and lethal increase in temperature on and within the items being sterilized.
Temperature, Not Pressure, Is the Killing Agent
While pressure is essential, it is merely the tool used to achieve the necessary temperature. The high temperature of the steam—typically 121°C or higher—is what denatures the essential proteins and enzymes of microorganisms, killing them.
The pressure itself does not kill the microbes; it enables the water to remain steam at a lethal temperature.
The Critical Role of Saturated Steam
For sterilization to succeed, the chamber must be filled with saturated steam, meaning all air has been removed. Air pockets act as an insulating barrier, preventing steam from making direct contact with surfaces.
If steam cannot touch a surface, it cannot transfer heat, and that area will not be sterilized. This is why preventing air entrapment is one of the most critical aspects of proper autoclave operation.
The Anatomy of a Standard Autoclave
Every component of an autoclave is engineered to create, maintain, and monitor the high-pressure steam environment needed for sterilization.
The Pressure Chamber and Door
The core of the unit is the pressure chamber, a vessel with robust walls (often an inner chamber and an outer jacket) designed to safely contain high-pressure steam. The door creates an airtight seal, which is crucial for building and maintaining pressure.
The Steam Generation System
An autoclave needs a source of steam. Some units have a built-in electrical heater or steam generator that boils water in a reservoir. Others connect to a central steam supply within a facility.
The Control and Monitoring System
This system is the brain of the autoclave. It includes a pressure gauge to display internal pressure and controls to set the time and temperature for the cycle. Modern autoclaves often have sophisticated software for precise cycle management and logging.
The Air and Condensate Removal System
Perhaps the most critical and least understood component is the system that removes air. A thermostatic trap or valve allows cooler air and condensed water to exit the chamber while trapping the hotter steam inside.
More advanced autoclaves use a vacuum system to actively pull all air out of the chamber before introducing steam, ensuring complete steam penetration from the start.
The Safety Valve
A non-negotiable component, the safety valve is a mechanical failsafe designed to automatically release pressure if it exceeds a safe limit, preventing catastrophic failure of the chamber.
Understanding the Trade-offs: Common Pitfalls in Autoclaving
Meeting the standard temperature and time does not guarantee sterilization if the process is executed improperly. Understanding these common failure points is essential.
Improper Loading and Air Pockets
The most common cause of sterilization failure is incorrect loading. Packing items too densely or using sealed containers prevents steam from circulating and making contact with all surfaces.
Air becomes trapped, creating cold spots where microorganisms can easily survive the cycle.
Mismatched Cycle Times
The standard 15-60 minute duration is a wide range because the required time depends entirely on the load. A small load of unwrapped metal instruments may be sterilized in 15 minutes.
However, a large, dense load or a flask of liquid requires a much longer cycle time to ensure the center of the load reaches the target temperature. One cycle time does not fit all applications.
Wet Packs and Recontamination
A successful cycle includes a drying phase. If items are removed from the autoclave while still wet, the moisture can act as a wick, pulling bacteria and viruses from the surrounding air through the packaging and recontaminating the sterile instruments.
Applying the Standard for Effective Sterilization
To ensure your process is effective, match the autoclave's function to your specific goal.
- If your primary focus is routine sterilization of solid instruments: Use the standard 121°C/15 psi cycle and ensure items are loosely packed to prevent air pockets and allow for complete steam contact.
- If your primary focus is sterilizing liquids or laboratory media: Use a specific "liquid" cycle, which uses a slower exhaust phase to prevent the liquids from boiling over as the pressure is released.
- If your primary focus is sterilizing porous materials (e.g., gowns or dense packs): Prioritize an autoclave with a pre-vacuum cycle to guarantee that all air is removed and steam can penetrate the entire load.
Ultimately, effective sterilization comes from understanding the principles of moist heat, not just the settings on the machine.
Summary Table:
| Key Autoclave Standard Parameter | Purpose & Importance |
|---|---|
| Temperature: 121°C (250°F) | The lethal temperature that denatures microbial proteins. |
| Pressure: 15 psi | The pressure required to achieve and maintain 121°C steam. |
| Time: 15-60 minutes | Duration needed for heat to penetrate and kill all organisms, including spores. |
| Agent: Saturated Steam | Moist heat transfers energy efficiently; air must be removed for steam contact. |
Ensure Your Lab Meets Sterilization Standards with KINTEK
Achieving true sterilization is critical for the integrity of your research, diagnostics, and production. Don't leave it to chance. KINTEK specializes in high-performance autoclaves and lab equipment designed to deliver reliable, consistent results.
We help you:
- Prevent contamination with autoclaves that ensure complete air removal and steam penetration.
- Save time and resources by selecting the right cycle for your specific materials—solids, liquids, or porous loads.
- Maintain compliance with equipment that meets rigorous industry standards.
Ready to optimize your sterilization process? Let our experts guide you to the perfect solution for your laboratory's needs.
Contact KINTEK today for a consultation and achieve peace of mind with every cycle.
Visual Guide
Related Products
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast Laboratory Autoclave Sterilizer 20L 24L for Lab Use
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
People Also Ask
- Why is the standard autoclave temperature set to 121? The Science of Effective Sterilization
- What is the necessity of using an autoclave for pre-treating culture media? Ensure Accurate Ag2O/TiO2 Testing
- What is the normal temperature of an autoclave? Achieve Sterile Confidence with Precise Control
- What critical environmental conditions does a laboratory autoclave provide for evaluating wear resistance? - KINTEK
- What are the standard operating parameters for an autoclave? Master Temperature, Pressure, and Time for Sterilization



















