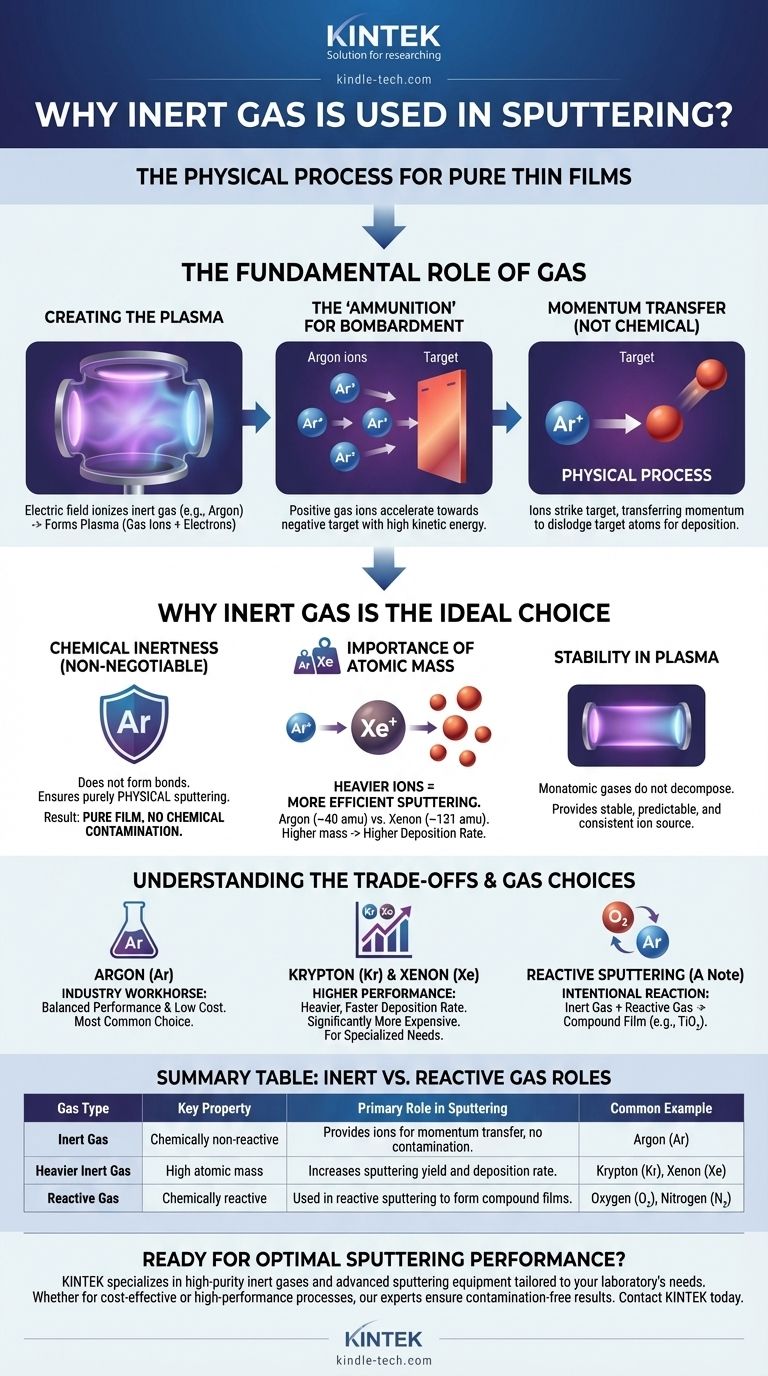
In short, inert gas is used in sputtering because it is chemically non-reactive and has the ideal physical properties to act as the "ammunition" for the process. It provides a stable source of ions that can be accelerated to physically bombard a target, knocking atoms loose for deposition without causing unwanted chemical reactions that would contaminate the resulting thin film.
Sputtering is a fundamentally physical process, not a chemical one. The primary role of an inert gas like argon is to provide a heavy, non-reactive projectile (an ion) that transfers momentum to a target, ensuring the material that is deposited is identical in composition to the material that was removed.
The Fundamental Role of Gas in Sputtering
To understand why inert gas is critical, you must first understand the core mechanics of the sputtering process. The gas is not a passive bystander; it is the essential medium that enables the entire operation.
Creating the Plasma
The process begins by introducing a small amount of gas into a vacuum chamber. A strong electric field is then applied, which energizes the gas atoms and strips them of electrons.
This creates plasma, a highly ionized state of matter consisting of positive gas ions and free electrons. This plasma is the engine of the sputtering process.
The "Ammunition" for Bombardment
The target material (the source of the film) is given a negative electrical charge. This causes the positively charged gas ions from the plasma to accelerate aggressively towards the target.
These ions strike the target surface with significant kinetic energy.
Momentum Transfer, Not Chemical Reaction
The goal of this bombardment is momentum transfer. Think of it as a microscopic game of billiards. The incoming gas ion is the cue ball, and its objective is to hit the atoms on the target's surface with enough force to knock them loose.
These dislodged target atoms then travel through the chamber and deposit onto a substrate, forming a thin, uniform film.
Why Inert Gas is the Ideal Choice
While any gas can be ionized to form a plasma, using a non-inert gas would fundamentally corrupt the process. The unique properties of inert gases like Argon (Ar), Krypton (Kr), and Xenon (Xe) make them uniquely suited for this task.
Chemical Inertness is Non-Negotiable
This is the most critical factor. Inert gases do not readily form chemical bonds with other elements.
If you were to use a reactive gas like oxygen or nitrogen, the ions would not only knock target atoms loose but also react with them. This would form unintended compounds (like oxides or nitrides) on the target surface and in the final film.
Using an inert gas ensures that the sputtering process remains purely physical, guaranteeing the deposited film is chemically identical to the target material.
The Importance of Atomic Mass
The efficiency of the momentum transfer—and thus the sputtering rate—is directly related to the mass of the bombarding ion.
A heavier ion striking a target atom transfers more energy than a lighter one, increasing the probability of dislodging a target atom. This is why heavier inert gases result in higher deposition rates.
Argon (atomic mass ~40 amu) is the most common choice, but for even higher efficiency, heavier gases like Krypton (~84 amu) or Xenon (~131 amu) can be used.
Stability in the Glow Discharge
Inert gases are monatomic and do not decompose under the intense energy of the plasma. This provides a stable, predictable, and consistent source of ions for bombarding the target, leading to a controlled and repeatable deposition process.
Understanding the Trade-offs
While the principle is straightforward, the choice of a specific inert gas involves balancing performance against cost.
Argon: The Industry Workhorse
Argon is the most widely used sputtering gas. It offers an excellent balance between a reasonably high atomic mass for efficient sputtering and a relatively low cost due to its abundance (it makes up ~1% of Earth's atmosphere).
Heavier Gases: For Higher Performance
Krypton and Xenon are significantly heavier than argon and will produce a higher sputter yield (more target atoms dislodged per ion). This leads to faster deposition rates.
However, these gases are much rarer and therefore significantly more expensive. They are typically reserved for specialized processes where maximum throughput is critical and cost is a secondary concern.
A Note on Reactive Sputtering
It is important to distinguish physical sputtering from reactive sputtering. In reactive sputtering, a reactive gas (like oxygen or nitrogen) is intentionally added to the inert gas flow.
The goal here is different: to form a compound film on the substrate. For example, by sputtering a titanium (Ti) target in an argon/oxygen plasma, you can deposit a titanium dioxide (TiO₂) film. The inert argon still does most of the physical sputtering, while the oxygen reacts with the sputtered titanium atoms to form the desired compound.
Making the Right Choice for Your Goal
Your choice of gas is dictated entirely by the desired outcome of your deposition process.
- If your primary focus is depositing a pure, uncontaminated film: Using a high-purity inert gas is mandatory to prevent any chemical reactions with the target or substrate.
- If your primary focus is maximizing deposition rate and efficiency: Choosing a heavier inert gas like Krypton or Xenon will increase your sputter yield, but at a significantly higher operational cost.
- If your primary focus is a cost-effective, general-purpose process: Argon is the industry standard, providing a reliable balance of performance and affordability for the vast majority of applications.
- If your primary focus is creating a compound film (e.g., an oxide or nitride): You will use reactive sputtering, which involves a carefully controlled mixture of an inert gas and a reactive gas.
Ultimately, the inert gas is the critical tool that enables the controlled, physical transfer of material from a source target to your substrate.
Summary Table:
| Gas Type | Key Property | Primary Role in Sputtering | Common Example |
|---|---|---|---|
| Inert Gas | Chemically non-reactive | Provides ions for momentum transfer without contamination | Argon (Ar) |
| Heavier Inert Gas | High atomic mass | Increases sputtering yield and deposition rate | Krypton (Kr), Xenon (Xe) |
| Reactive Gas | Chemically reactive | Used in reactive sputtering to form compound films | Oxygen (O₂), Nitrogen (N₂) |
Ready to achieve pure, high-quality thin films with optimal sputtering performance?
KINTEK specializes in providing high-purity inert gases and advanced sputtering equipment tailored to your laboratory's specific needs. Whether you require cost-effective argon for general processes or high-performance krypton/xenon for maximum deposition rates, our experts will help you select the ideal solution for contamination-free results.
Contact KINTEK today to discuss your sputtering requirements and enhance your thin film deposition process!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- VHP Sterilization Equipment Hydrogen Peroxide H2O2 Space Sterilizer
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
People Also Ask
- What is plasma activated chemical vapour deposition method? A Low-Temperature Solution for Advanced Coatings
- What are the uses of PECVD? A Guide to Low-Temperature Thin-Film Deposition
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- What is an example of PECVD? RF-PECVD for High-Quality Thin Film Deposition
- What is the difference between PECVD and sputter? Choose the Right Thin-Film Deposition Method



















