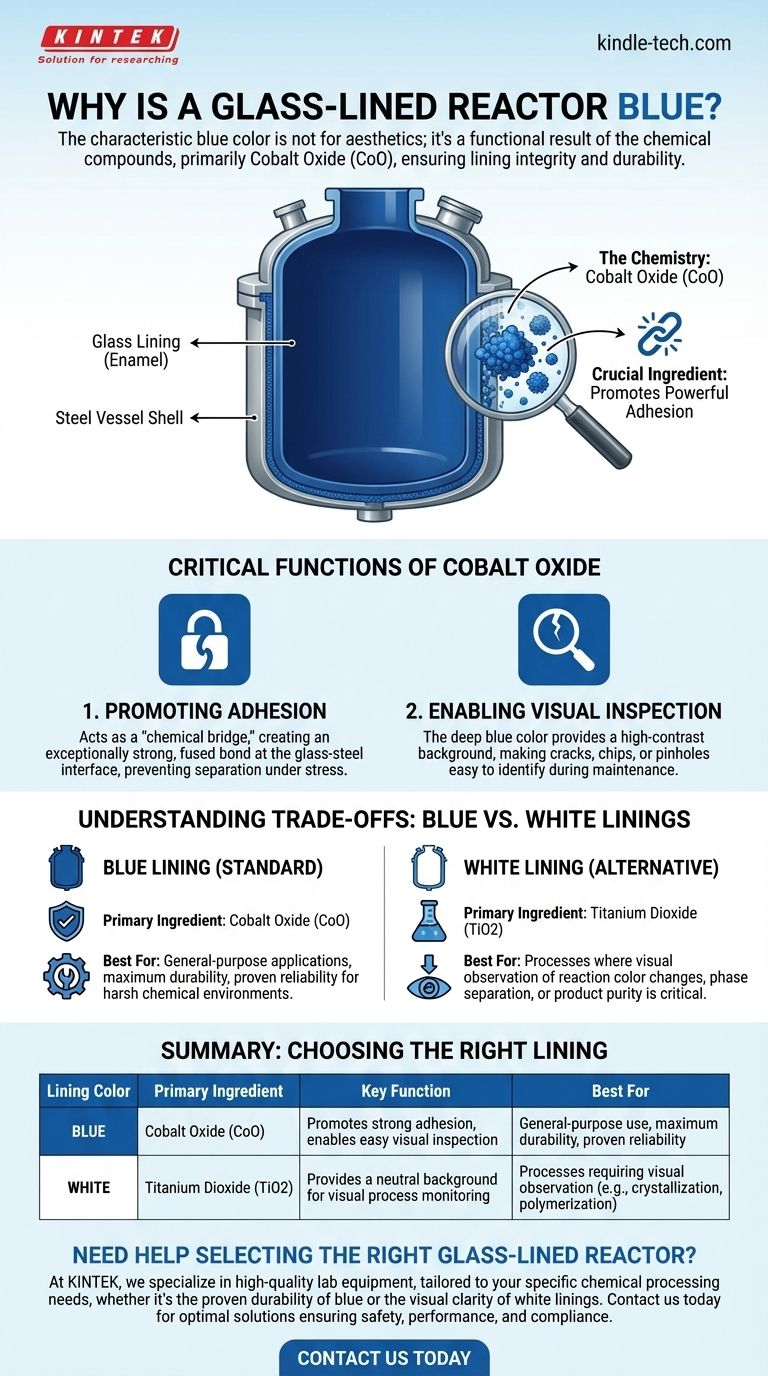
The characteristic blue color of a glass-lined reactor is not for aesthetics; it is a direct result of the specific chemical compounds used in the enamel formulation. The primary reason for this color is the addition of cobalt oxide (CoO), a crucial ingredient that ensures the lining's integrity and durability by promoting a powerful bond between the glass and the steel shell of the reactor.
The blue color signifies a fundamental engineering choice. It arises from using cobalt oxide, an essential agent that chemically fuses the glass lining to the steel vessel, guaranteeing mechanical strength and simplifying visual safety inspections.

The Chemistry Behind the Color
The glass lining in a reactor is a specialized form of enamel, a vitreous material fired at high temperatures to create a non-porous, corrosion-resistant surface. The final properties of this lining, including its color, are dictated by its chemical makeup.
The Role of Cobalt Oxide (CoO)
The deep blue hue is imparted by the deliberate inclusion of cobalt oxide in the raw enamel mixture, known as "frit." Cobalt has been used for centuries to create rich blue colors in glass and ceramics, and its function here is rooted in this same principle.
More Than Just a Pigment
Unlike a simple paint or dye, cobalt oxide is a functional component of the enamel. Its presence is critical to the manufacturing process and the final performance of the reactor lining, serving purposes far more important than appearance.
Why Cobalt Blue is the Industry Standard
The prevalence of blue glass-lined reactors is a testament to a time-tested formulation that prioritizes safety, longevity, and reliability in harsh chemical environments.
Critical Function 1: Promoting Adhesion
The single most important reason for using cobalt oxide is its role as an adhesion promoter. During the high-temperature firing process, the cobalt oxide creates an intermediate oxide layer at the interface between the molten glass and the carbon steel substrate.
This layer acts as a "chemical bridge," creating an exceptionally strong, fused bond. Without it, the glass and steel—two very different materials with different rates of thermal expansion—would be far more likely to separate or delaminate under the stress of temperature and pressure changes.
Critical Function 2: Enabling Visual Inspection
The deep, uniform blue color provides a high-contrast background that makes it easy to perform visual inspections. Any cracks, chips, pinholes, or areas of wear will appear as starkly different colors, allowing maintenance teams to identify potential points of failure before they lead to a catastrophic breach and vessel corrosion.
Historical Precedent and Reliability
Cobalt-based enamel formulations are the original standard for glass-lined steel. They have a multi-decade track record of proven performance and reliability across a vast range of chemical processes, making blue the default, trusted choice for general-purpose applications.
Understanding the Trade-offs: Blue vs. White Linings
While blue is the most common color, it is not the only option. White linings are also widely available and serve specific process needs.
The Case for White Linings
White glass linings are created using titanium dioxide (TiO2) as an opacifying agent instead of cobalt oxide. These formulations are specifically designed for applications where the visual observation of the process itself is critical.
Application-Specific Choices
A white lining is preferred for processes such as crystallization, polymerization, or reactions where monitoring color change, phase separation, or product purity in real-time is necessary. The blue color would obscure these subtle visual cues.
Modern Formulation Parity
Historically, blue linings were considered to have superior adhesion. However, modern manufacturing techniques and enamel formulations have significantly improved the performance of white linings. Today, high-quality white linings offer reliability comparable to their blue counterparts, making the choice primarily one of application requirement rather than durability.
Making the Right Choice for Your Process
Your choice of lining color should be driven by your operational priorities and specific process requirements, not by tradition or aesthetics alone.
- If your primary focus is maximum durability and proven reliability for general use: The standard cobalt-blue lining is the default, time-tested choice for a wide range of corrosive applications.
- If your primary focus is observing the color or state of your reaction mixture: A white, titanium-based lining is the correct choice for processes where visual monitoring is a critical control parameter.
- If you are concerned about trace metal leaching for high-purity products (e.g., cGMP): You must consult the manufacturer's specifications for cobalt or titanium leaching to ensure compliance with your purity standards.
Ultimately, the color of a glass-lined reactor is a direct indicator of its underlying chemical engineering, designed for safety and performance.
Summary Table:
| Lining Color | Primary Ingredient | Key Function | Best For |
|---|---|---|---|
| Blue | Cobalt Oxide (CoO) | Promotes strong adhesion, enables easy visual inspection | General-purpose use, maximum durability, proven reliability |
| White | Titanium Dioxide (TiO2) | Provides a neutral background for visual process monitoring | Processes requiring visual observation (e.g., crystallization, polymerization) |
Need help selecting the right glass-lined reactor for your specific process?
At KINTEK, we specialize in providing high-quality lab equipment, including glass-lined reactors tailored to your chemical processing needs. Whether you require the proven durability of a cobalt-blue lining or the visual clarity of a white lining for sensitive reactions, our experts can guide you to the optimal solution.
Contact us today to discuss your application and ensure you get a reactor that guarantees safety, performance, and compliance with your purity standards.
Visual Guide
Related Products
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How does a high-pressure hydrothermal autoclave facilitate the synthesis of BiVO4@PANI nanocomposites? Unlock Precision.
- What is the significance of the hydrothermal environment in HA preparation? Optimize Mesoporous Structure and Purity
- What environment does a PTFE-lined autoclave provide for TiO2-GQD synthesis? Achieve Superior Nanocomposite Results
- What is the role of a PTFE-lined stainless steel high-pressure autoclave in ZrW2O8 synthesis? Achieve High Purity
- Which critical experimental conditions does a high-pressure autoclave provide? Optimize Mixed Sulfide Leaching



















