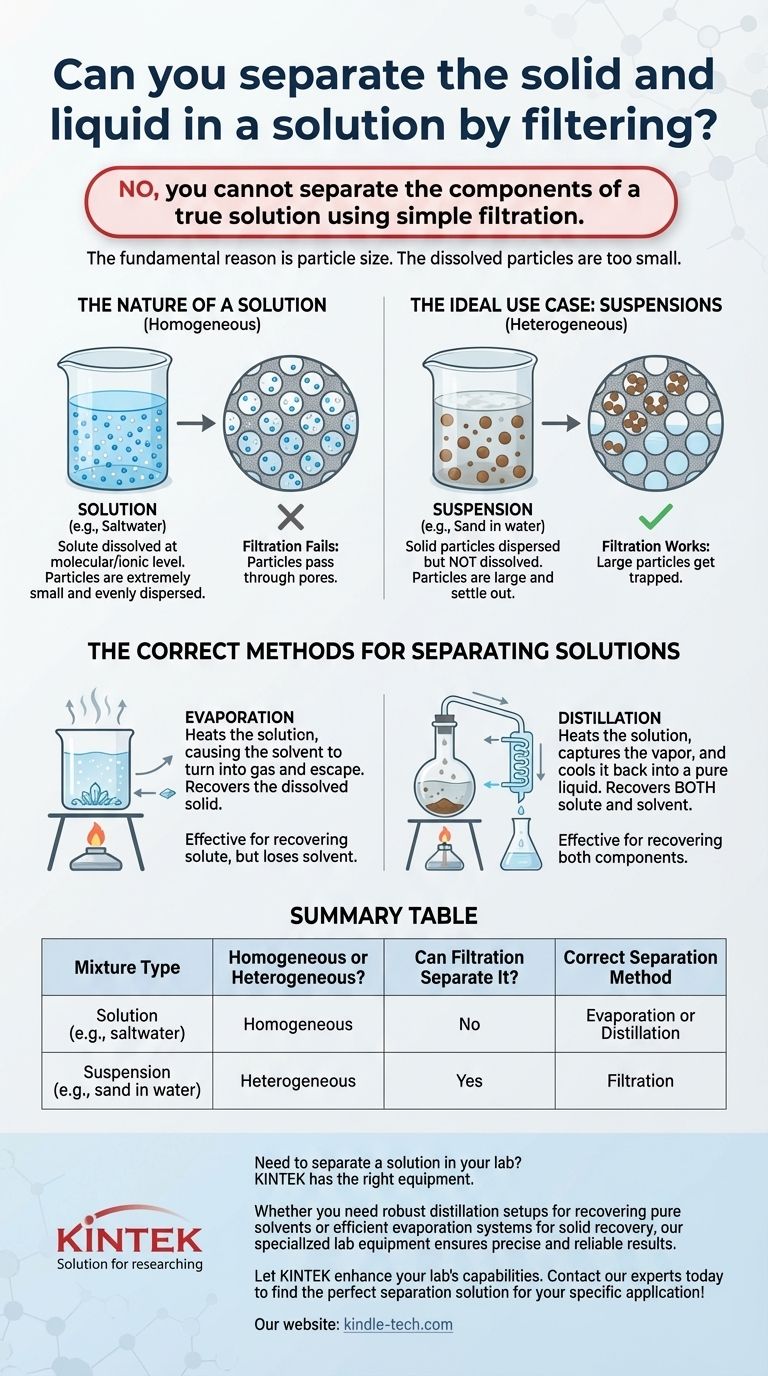
No, you cannot separate the components of a true solution using simple filtration. The fundamental reason is particle size. In a solution, the solid (solute) is dissolved at the molecular or ionic level, meaning its particles are far too small to be captured by the pores of a standard filter. The dissolved particles will pass through the filter along with the liquid solvent.
The effectiveness of a separation technique is dictated by the physical properties of the mixture. Filtration is designed for heterogeneous mixtures like suspensions, where undissolved particles are large enough to be physically blocked, not for homogeneous mixtures like solutions, where components are mixed at the molecular level.

Why Filtration Fails with Solutions
To understand this limitation, we must first distinguish between a solution and other types of mixtures. The method must match the mixture.
The Nature of a Solution
A solution is a homogeneous mixture, meaning the components are uniformly distributed. It consists of a solute (the substance that dissolves) and a solvent (the substance it dissolves in).
When a solute like salt dissolves in a solvent like water, its crystalline structure breaks apart. The individual salt ions become completely surrounded by water molecules, dispersing evenly throughout the liquid.
At this point, you can no longer see the individual solute particles. They are not floating; they are fully integrated into the solvent at a molecular scale.
The Mechanics of Filtration
Filtration is a purely physical separation method. Think of it as a sieve or a screen.
Filter paper contains microscopic pores of a specific size. When you pour a liquid mixture through it, particles larger than the pores get trapped, while the liquid and anything small enough to fit through the pores passes.
The problem is a massive scale mismatch. The ions of dissolved salt are thousands of times smaller than the pores in typical filter paper. Trying to filter a salt solution is like trying to catch sand with a chain-link fence—it's simply the wrong tool for the job.
When Filtration Is the Right Tool
Filtration is an extremely effective and common technique when used for the correct type of mixture.
The Ideal Use Case: Suspensions
The ideal candidate for filtration is a suspension. This is a heterogeneous mixture where solid particles are dispersed in a liquid but are not dissolved.
A classic example is sand in water. The sand particles are visibly distinct and will eventually settle out due to gravity.
Because these particles are much larger than the filter's pores, they are easily captured, allowing the clear liquid to pass through. This is the core principle behind coffee makers, water purifiers, and countless industrial processes.
The Correct Methods for Separating Solutions
If filtration won't work, you must use a method that exploits a different physical property, such as the boiling points of the components.
Evaporation
This is the simplest method for recovering a dissolved solid from a liquid solvent.
By heating the solution (e.g., saltwater), you increase the energy of the solvent molecules until they turn into a gas and escape, or evaporate. The solid solute, which has a much higher boiling point, is left behind.
This method is effective, but you lose the solvent to the atmosphere.
Distillation
Distillation allows you to recover both the solute and the solvent.
The process involves boiling the solution, but instead of letting the vapor escape, you capture it. This vapor is then channeled through a cooled tube (a condenser), which causes it to turn back into a pure liquid.
This works because the solvent (like water) has a lower boiling point than the dissolved solid (like salt). The pure, condensed solvent is collected in a separate container, leaving the original solid behind.
How to Choose the Right Separation Method
Your choice depends entirely on the nature of your mixture and which components you need to recover.
- If you have undissolved solid particles in a liquid (a suspension): Use filtration to efficiently separate the solid from the liquid.
- If you want to recover a dissolved solid from a liquid (a solution): Use evaporation, but be prepared to lose the liquid solvent.
- If you want to recover the liquid solvent from a solution (or both components): Use distillation to separate and collect the pure liquid.
Understanding the fundamental difference between a suspension and a solution is the key to selecting the correct tool for the task.
Summary Table:
| Mixture Type | Homogeneous or Heterogeneous? | Can Filtration Separate It? | Correct Separation Method |
|---|---|---|---|
| Solution (e.g., saltwater) | Homogeneous | No | Evaporation or Distillation |
| Suspension (e.g., sand in water) | Heterogeneous | Yes | Filtration |
Need to separate a solution in your lab? Filtration won't work, but KINTEK has the right equipment for the job. Whether you need robust distillation setups for recovering pure solvents or efficient evaporation systems for solid recovery, our specialized lab equipment ensures precise and reliable results.
Let KINTEK enhance your lab's capabilities. Contact our experts today to find the perfect separation solution for your specific application!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
People Also Ask
- What is the purpose of an evaporator? The Key Component That Creates Cooling
- What is the purpose of laboratory freeze drying? Preserve Sensitive Drugs & Biologics for Stability
- What problems should be avoided when using a lyophilizer? Prevent Product Collapse and Equipment Overload
- How does freeze drying extend the shelf life of pharmaceutical products? Preserve Potency and Stability for Years
- Which industries commonly use lab freeze dryers? Preserve Sensitive Materials with Lyophilization



















