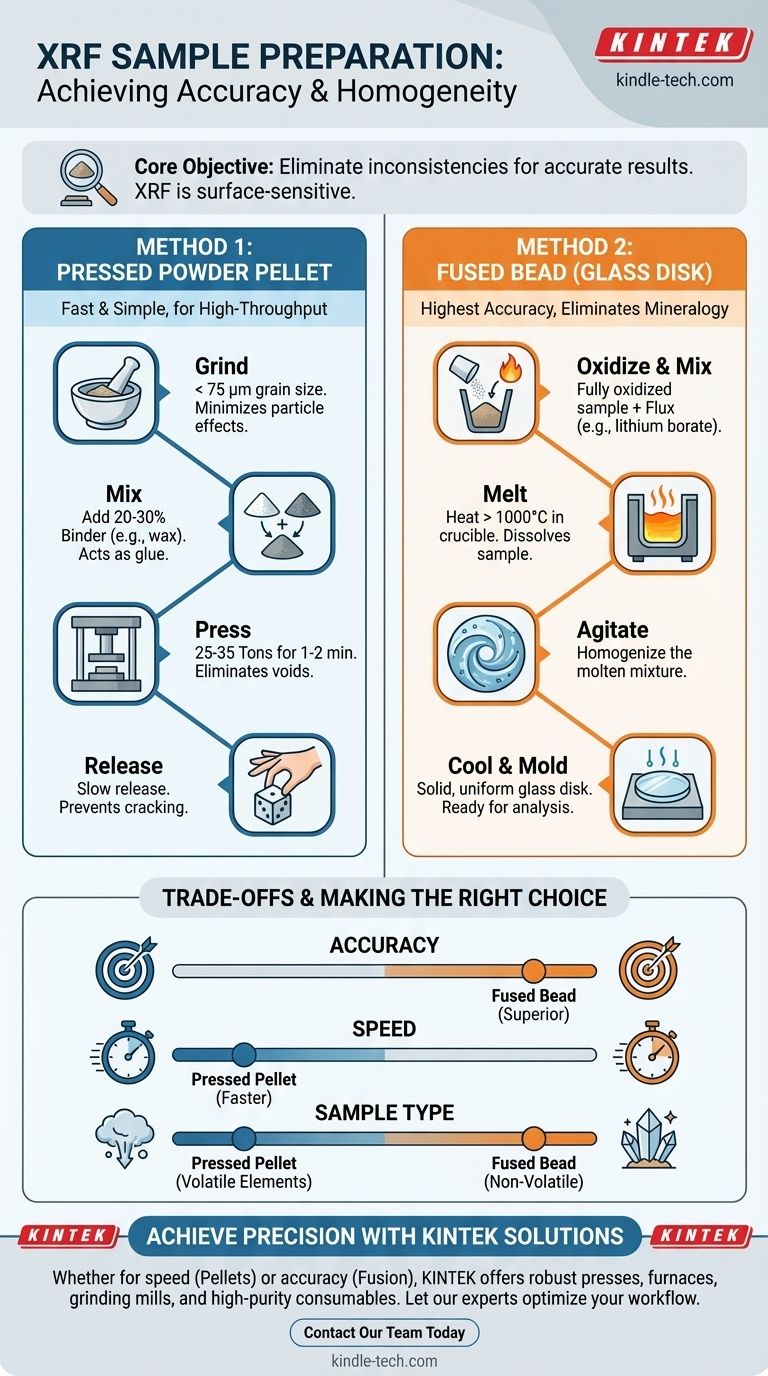
Preparing a sample for X-ray Fluorescence (XRF) analysis is a critical step that dictates the accuracy of your results. The two primary methods are compressing the material into a solid pressed powder pellet or dissolving it in a flux at high temperature to create a homogeneous fused glass disk, also known as a fused bead. Each method is designed to present a perfectly flat, uniform, and representative surface to the X-ray beam.
Your core objective in XRF sample preparation is to eliminate physical and chemical inconsistencies within the sample. Because XRF is a surface-sensitive technique, any variation in particle size, density, or mineral composition will directly compromise the quality of your data.

The Principle: Homogeneity is Everything
XRF analysis relies on bombarding a sample with X-rays and measuring the secondary X-rays that are emitted. The intensity of these secondary X-rays is proportional to the concentration of each element. This principle only holds true if the sample surface is perfectly uniform.
The Problem of Heterogeneity
An unprepared sample, like a raw powder or rock fragment, has countless variables that distort results. These include different mineral phases, variations in particle size, and empty spaces (voids) between grains. These effects introduce significant error, particularly for lighter elements whose X-rays are less energetic.
The Solution: Create a Perfect Surface
Both pressed pellets and fused beads solve this problem by transforming a heterogeneous powder into a solid with a smooth, uniform surface. This ensures that the X-ray beam interacts with a sample that is a true representation of the bulk material.
Method 1: The Pressed Powder Pellet
This is the most common method for its speed and simplicity. It involves grinding the sample into a fine powder and compressing it under high pressure to form a durable disc.
Step 1: Grinding the Sample
The first step is to reduce the sample to a fine, consistent powder, ideally with a grain size of less than 75 micrometers. This minimizes what are known as "particle size effects," where larger grains can disproportionately absorb or scatter the X-rays, skewing the results.
Step 2: Mixing with a Binder
The fine powder is then mixed with a binding agent, such as a cellulose or wax blend. This binder acts as a glue to hold the sample particles together. A typical ratio is 20-30% binder to sample, though this can be optimized. The binder helps create a strong, durable pellet that won't crumble during handling.
Step 3: Applying Pressure
The sample-binder mixture is placed into a die and compressed at 25 to 35 tons of pressure for one to two minutes. This immense pressure forces the particles together, recrystallizes the binder, and critically, eliminates void spaces that can weaken the signal from light elements.
Step 4: Releasing Pressure Slowly
Pressure must be released gradually from the die. Releasing it too quickly can cause the pellet's surface to crack or flake, ruining the perfect analytical surface you just created and requiring you to start over.
Method 2: The Fused Bead (Glass Disk)
Fusion is a more complex but often more accurate method. It completely eliminates particle size and mineralogical effects by dissolving the sample into a glass disk.
The Fusion Process
The sample, which must be fully oxidized, is mixed with a solvent chemical called a flux (e.g., a lithium borate). This mixture is placed in a platinum, zirconium, or graphite crucible and heated to a high temperature (often over 1000°C) until it melts completely.
Creating the Glass Disk
The molten liquid is agitated to ensure it is perfectly mixed. Finally, it is poured into a mold and cooled to form a solid, transparent, and almost perfectly homogeneous glass disk ready for analysis.
Understanding the Trade-offs: Pellet vs. Bead
Choosing the right method requires balancing your need for accuracy with practical considerations like speed, cost, and sample type.
Accuracy and Precision
The fused bead method is generally superior for accuracy. By completely destroying the original crystal structure of the sample, it eliminates virtually all mineralogical and particle size effects, which is the largest source of error in the pressed pellet method.
Speed and Simplicity
Pressed pellets are significantly faster and simpler to prepare. The process requires less specialized equipment (a grinder and a press) and can often be completed in minutes, making it ideal for high-throughput environments like quality control labs.
Sample Composition
Pressed pellets are necessary for volatile elements (like sodium or sulfur) that would be lost during the high-temperature fusion process. Fusion is only suitable for non-volatile materials.
Making the Right Choice for Your Goal
Your analytical requirements should dictate your preparation method.
- If your primary focus is high-throughput screening or routine process control: The speed and simplicity of pressed powder pellets is your best choice.
- If your primary focus is the highest possible accuracy for research or material certification: The fused bead method provides the gold standard by eliminating the most significant sources of error.
- If your primary focus is analyzing samples containing volatile elements: You must use the pressed powder pellet method to avoid losing those elements at high temperatures.
Ultimately, meticulous sample preparation is the foundation upon which all accurate XRF analysis is built.
Summary Table:
| Method | Key Advantage | Ideal For | Key Limitation |
|---|---|---|---|
| Pressed Powder Pellet | Speed, simplicity, cost-effectiveness | High-throughput screening, samples with volatile elements (e.g., S, Na) | Susceptible to mineralogical/particle size effects |
| Fused Bead (Glass Disk) | Highest accuracy, eliminates mineralogical effects | Research, material certification, ultimate precision | Not suitable for volatile elements; more complex process |
Achieve precise and reliable XRF analysis with KINTEK's sample preparation solutions.
The right preparation method is critical for your lab's success. Whether you need the speed of pressed pellets for quality control or the superior accuracy of fusion for R&D, KINTEK has the robust equipment and consumables—including presses, fusion furnaces, grinding mills, and high-purity binders & fluxes—to meet your specific laboratory needs.
Let our experts help you optimize your workflow. Contact our team today to discuss your application and find the perfect solution!
Visual Guide
Related Products
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
People Also Ask
- What role does a laboratory hydraulic press play in the preparation of solid electrolyte pellets? Ensure Data Accuracy
- What is the role of a laboratory hydraulic press and pellet die in FTIR? Optimize BiVO4@PANI Characterization
- Why is a laboratory hydraulic press used for MOF-CGC pellets? Maximize Density and Encapsulation Quality
- How are laboratory hydraulic presses used in catalyst preparation? Key Steps for Pelletizing Heterogeneous Catalysts
- Why is a laboratory hydraulic press essential for Ca3Co4O9 pelletizing? Optimize Pre-Sintering Mass Transport



















