Sterilization without an autoclave is not only possible but is the required standard for many materials. The primary methods involve using low-temperature chemical processes like ethylene oxide or vaporized hydrogen peroxide, specialized radiation techniques, or physical removal through sterile filtration, each chosen based on the material's specific properties and sensitivities.
Choosing a sterilization method is not about finding a direct replacement for an autoclave. It is about understanding the fundamental principle of matching the sterilization technique—be it heat, chemical, or radiation—to the material you need to sterilize.
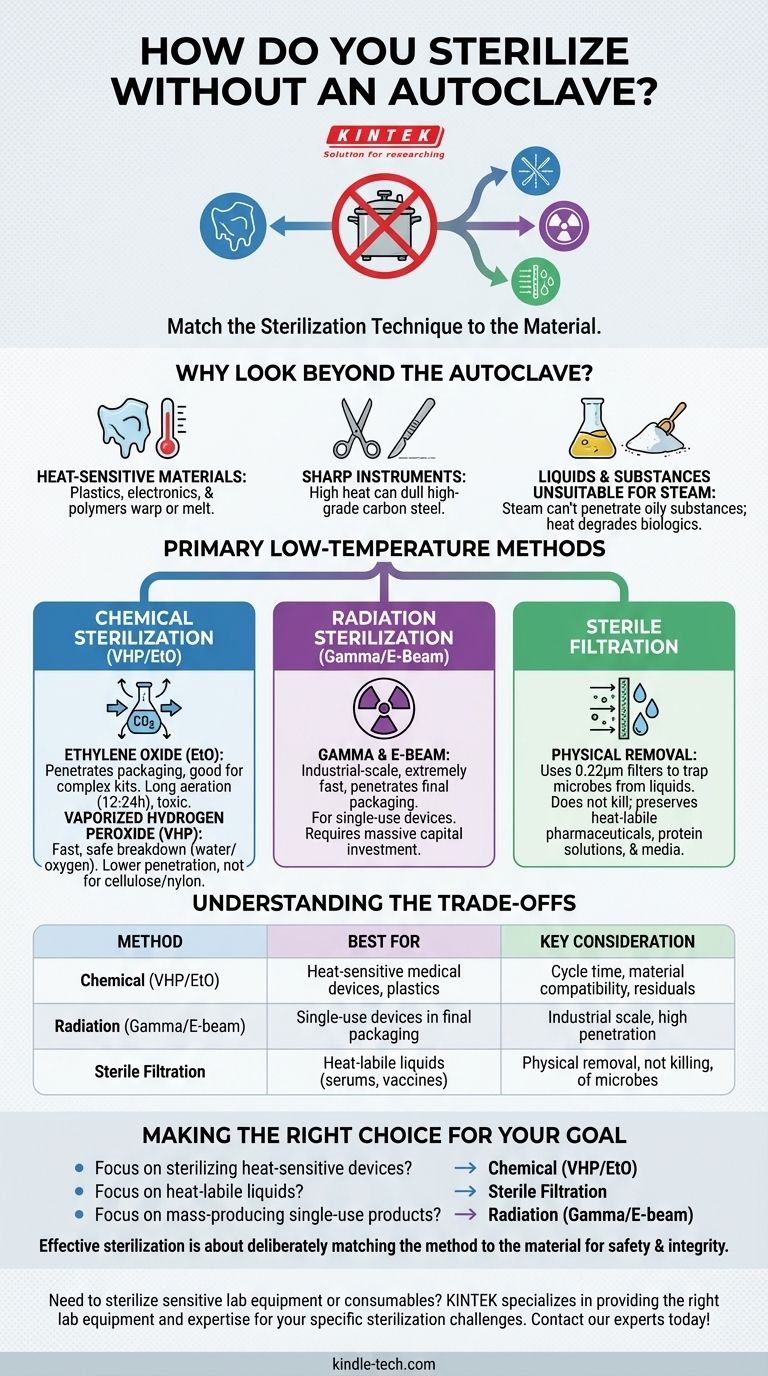
Why Look Beyond the Autoclave?
While the autoclave is a cornerstone of sterilization using pressurized steam and heat, its very mechanism makes it unsuitable for a wide range of materials and substances. Understanding these limitations is the first step in selecting the correct alternative.
Heat-Sensitive Materials
Many modern medical devices, lab equipment, and electronics are made from polymers or plastics that would melt, warp, or be destroyed at the high temperatures of an autoclave. Autoclaves are fundamentally incompatible with most plastics and sensitive electronic components.
Sharp Instruments
High-grade carbon steel instruments, such as scalpels and certain scissors, can become dull when exposed to the high heat and moisture inside an autoclave. This compromises the instrument's primary function and integrity.
Liquids and Substances Unsuitable for Steam
Oily substances and powders do not mix with water, so steam cannot penetrate them effectively, rendering autoclave sterilization useless. Furthermore, heat-sensitive biological solutions like vaccines, serums, or certain proteins will degrade and lose their efficacy when subjected to intense heat.
Primary Low-Temperature Sterilization Methods
For materials that cannot withstand heat and moisture, low-temperature methods are the industry standard. These are not "lesser" options; they are specialized processes for specific applications.
Chemical Sterilization: Ethylene Oxide (EtO)
Ethylene Oxide is a toxic gas used for sterilizing heat- and moisture-sensitive devices. It can penetrate packaging materials, making it excellent for sterilizing complex, pre-packaged medical kits.
However, its toxicity requires lengthy aeration periods to remove residual gas, making the total cycle time very long (often 12-24 hours).
Chemical Sterilization: Vaporized Hydrogen Peroxide (VHP)
VHP sterilization is a faster and safer alternative to EtO for many heat-sensitive items. The process uses hydrogen peroxide vapor in a vacuum to sterilize devices.
Its primary advantage is that it breaks down into non-toxic water and oxygen, eliminating the need for long aeration cycles. However, it has lower penetration capability than EtO and is not suitable for materials like cellulose (paper) or nylon.
Radiation Sterilization: Gamma and E-Beam
This is a highly effective, industrial-scale method used primarily for single-use medical devices like syringes, catheters, and implants. High-energy gamma rays or electron beams (E-beams) penetrate products in their final sealed packaging.
Radiation sterilization is extremely fast and reliable but requires a massive capital investment in specialized, shielded facilities. It is not a method used in a typical lab or clinical setting but rather by large-scale manufacturers.
Sterilization for Liquids and Biologics
When the goal is to sterilize a heat-sensitive liquid, killing microorganisms with heat or chemicals is not an option. The solution is physical removal.
Sterile Filtration
This method uses a filter with a pore size small enough (typically 0.22 micrometers) to physically trap and remove bacteria and other microorganisms from a liquid as it passes through.
Sterile filtration does not kill microbes; it simply separates them from the fluid. It is the gold-standard method for sterilizing temperature-sensitive pharmaceuticals, protein solutions, and cell culture media without damaging the product.
Understanding the Trade-offs
No sterilization method is perfect for every situation. Choosing the right one requires balancing efficacy, material compatibility, safety, and cost.
Efficacy vs. Material Compatibility
The autoclave offers a very high level of sterility assurance but has poor material compatibility. Chemical and radiation methods offer excellent compatibility with sensitive polymers but may face challenges with complex device geometries or material density.
Cycle Time and Throughput
Autoclaves offer relatively fast cycles (30-60 minutes). VHP is also quite fast. In contrast, EtO is extremely slow due to long aeration requirements. Radiation is the fastest for large, continuous batches.
Safety and Residuals
Steam from an autoclave leaves no toxic residue. VHP breaks down into harmless water and oxygen. EtO, however, is a known carcinogen, and strict protocols must be followed to ensure no harmful residuals remain on the device.
Making the Right Choice for Your Goal
Selecting a sterilization method requires a clear understanding of your material and your objective.
- If your primary focus is sterilizing heat-sensitive medical devices or plastics: Chemical methods like Vaporized Hydrogen Peroxide (for surface sterilization) or Ethylene Oxide (for deep penetration) are the correct choices.
- If your primary focus is sterilizing heat-labile liquids like serums or vaccines: Sterile filtration is the only appropriate method to preserve the integrity of the solution.
- If your primary focus is mass-producing single-use sterile products: Radiation sterilization (Gamma or E-beam) provides the highest efficiency and penetration for items in their final packaging.
- If your primary focus is robust, non-sensitive items like glassware or stainless steel tools: The autoclave remains the most accessible and reliable method.
Ultimately, effective sterilization is achieved by deliberately matching the method to the material, ensuring both safety and integrity.

Summary Table:
| Method | Best For | Key Consideration |
|---|---|---|
| Chemical (VHP/EtO) | Heat-sensitive medical devices, plastics | Cycle time, material compatibility, residuals |
| Radiation (Gamma/E-beam) | Single-use devices in final packaging | Industrial scale, high penetration |
| Sterile Filtration | Heat-labile liquids (serums, vaccines) | Physical removal, not killing, of microbes |
Need to sterilize sensitive lab equipment or consumables? KINTEK specializes in providing the right lab equipment and expertise for your specific sterilization challenges. Whether you're working with heat-sensitive polymers, electronics, or delicate biological solutions, we can help you select the optimal method to ensure both sterility and material integrity. Contact our experts today to discuss your application and find a safe, effective solution!
Visual Guide
Related Products
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Desktop Fast High Pressure Laboratory Autoclave Sterilizer 16L 24L for Lab Use
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Single Punch Electric Tablet Press Machine Laboratory Powder Tablet Punching TDP Tablet Press
- Wall Mounted Water Distillation Unit
People Also Ask
- How long does a typical autoclave run? Understand the Full Cycle for Safe Sterilization
- What is the function of a high-pressure static autoclave in biomass HTL? Optimize Your Biomass Conversion Research
- Why must Zircaloy-4 components undergo high-pressure autoclave steam oxidation? Ensure Critical Corrosion Resistance
- Why use PPL-Lined Autoclaves for Vanadium Dioxide Nanorods? Achieve Pure Crystallization at 280°C
- What is the primary role of a high-pressure autoclave in the solvothermal synthesis of ZIF-8? Optimize Your MOF Quality
- Why is a high-pressure autoclave essential for HMF conversion? Achieve Efficient Lignocellulose Synthesis
- What is the function of a high-pressure flowing autoclave in LWR testing for NITE-SiC? Simulating Reactor Conditions
- What is the application of retort machine in food industry? Ensuring Shelf-Stable Food Safety and Longevity



















