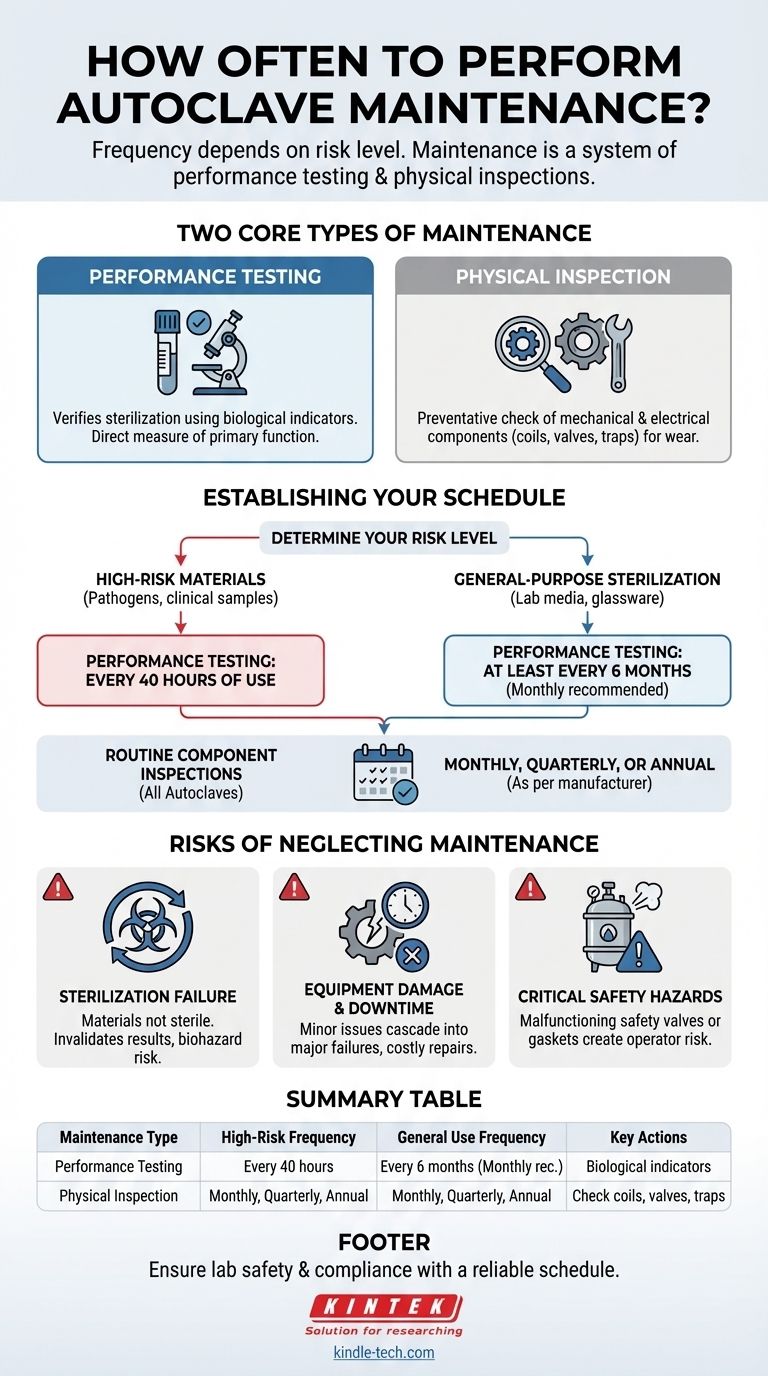
The required frequency for autoclave maintenance depends entirely on its use. For autoclaves inactivating high-risk materials like human pathogens or clinical samples, performance testing is required after every 40 hours of use. For all other materials, testing should occur at least every six months, while physical inspections of components should be conducted monthly, quarterly, or annually.
The key is to understand that "maintenance" is not a single event. It's a system of routine performance testing and periodic physical inspections, with the schedule for each determined by the level of risk associated with the materials you sterilize.

Differentiating Performance Testing from Physical Inspection
To create a reliable schedule, you must first distinguish between the two core types of autoclave maintenance. Each serves a different purpose and operates on a different timeline.
What is Performance Testing?
Performance testing verifies that the autoclave is achieving sterilization. This is typically done using biological indicators to confirm that the cycle effectively kills highly resistant microorganisms.
This is a direct measure of the machine's primary function and is the most frequent maintenance task.
What is Physical Inspection?
Physical inspection is a preventative check of the machine's mechanical and electrical components. This involves examining parts like heating coils, safety valves, and steam traps for wear and tear.
These inspections ensure the equipment remains in safe working order and help prevent costly damage from issues like poor water quality or particulate buildup in the steam.
Establishing Your Maintenance Schedule
Your specific schedule is dictated by what you sterilize. Higher-risk applications demand more frequent verification to ensure public and environmental safety.
For High-Risk Materials
If your autoclave is used to inactivate substances like human pathogens, blood, tissues, or clinical samples, you must conduct performance testing much more frequently.
The required interval is after every 40 hours of use. This ensures that every high-risk load is processed by a recently verified machine.
For General-Purpose Sterilization
For autoclaves used to sterilize other materials, such as lab media, glassware, or non-infectious waste, the testing schedule is less stringent.
Performance testing with a biological indicator must be conducted at least once every six months. Some institutions adopt a more proactive schedule of once per month to ensure consistent performance.
Routine Component Inspections
Regardless of use, all autoclaves require regular physical inspections to ensure longevity and safety.
These checks on key components should be performed on a monthly, quarterly, or annual basis as recommended by the manufacturer.
The Risks of Neglecting Maintenance
Skipping scheduled maintenance introduces significant risks that go far beyond simple machine failure. Understanding these consequences highlights the importance of a strict, documented protocol.
Sterilization Failure
This is the most critical risk. If the autoclave is not reaching the required temperature and pressure for the correct duration, your materials will not be sterile. For clinical or research labs, this can invalidate results, compromise experiments, and pose a direct biohazard.
Equipment Damage and Downtime
Minor issues, like a clogged steam trap or a failing heating coil, can cascade into major system failures if left unaddressed. Regular inspections catch these problems early, preventing expensive repairs and unplanned downtime that can halt operations.
Critical Safety Hazards
Autoclaves are pressurized vessels operating at high temperatures. A malfunctioning safety valve or a compromised door gasket can create a serious safety risk for operators. Routine inspections are essential to verify that these critical safety features are functioning correctly.
Creating the Right Schedule for Your Lab
Use your primary application to define your maintenance protocol. A documented schedule is essential for compliance, safety, and operational reliability.
- If your primary focus is inactivating biohazardous materials: Your most critical task is performance testing with a biological indicator after every 40 hours of operation.
- If your primary focus is sterilizing general lab supplies: You must perform biological indicator testing at least every six months, with monthly testing being the recommended best practice.
- If your primary focus is equipment safety and longevity: You must implement a recurring schedule for monthly, quarterly, and annual physical inspections of all key components, as specified by the manufacturer.
Ultimately, a robust maintenance schedule is the foundation of safe, effective, and reliable autoclave operation.
Summary Table:
| Maintenance Type | Frequency for High-Risk Use | Frequency for General Use | Key Components/Actions |
|---|---|---|---|
| Performance Testing | After every 40 hours of operation | At least every 6 months (monthly recommended) | Biological indicators to verify sterilization |
| Physical Inspection | Monthly, Quarterly, Annual (as per manufacturer) | Monthly, Quarterly, Annual (as per manufacturer) | Heating coils, safety valves, steam traps, door gaskets |
Ensure your lab's safety and compliance with a reliable autoclave maintenance schedule.
KINTEK specializes in laboratory equipment and consumables, providing the tools and support you need for effective sterilization. From biological indicators for performance testing to expert advice on creating a maintenance protocol, we help laboratories serving clinical, research, and industrial needs maintain peak performance and safety.
Contact KINTEK today to discuss your autoclave maintenance needs and ensure your equipment operates reliably.
Visual Guide
Related Products
- Portable High Pressure Laboratory Autoclave Steam Sterilizer for Lab Use
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
People Also Ask
- What other method besides autoclaving achieves sterilization? Compare Dry Heat, Chemical, and Radiation Options
- What is the role of the Bowie-Dick-Test in waste decontamination? Focus on Lab Efficiency & Compliance
- What is the procedure for performing a load validation for an autoclave? Ensure Compliance and Sterility Success
- How does temperature affect sterilization? Unlock the Science of Heat-Based Microbial Destruction
- Why is autoclave done at 121 C? The Science of Sterilizing Heat-Resistant Spores
- What is the purpose of autoclaving in the laboratory? Ensure Sterile Safety & Integrity
- Why are high-pressure autoclaves required for Zirconium alloy testing? Essential for Nuclear Environment Validation
- How long is the sterilization cycle in an autoclave? It's More Than Just 15 Minutes



















