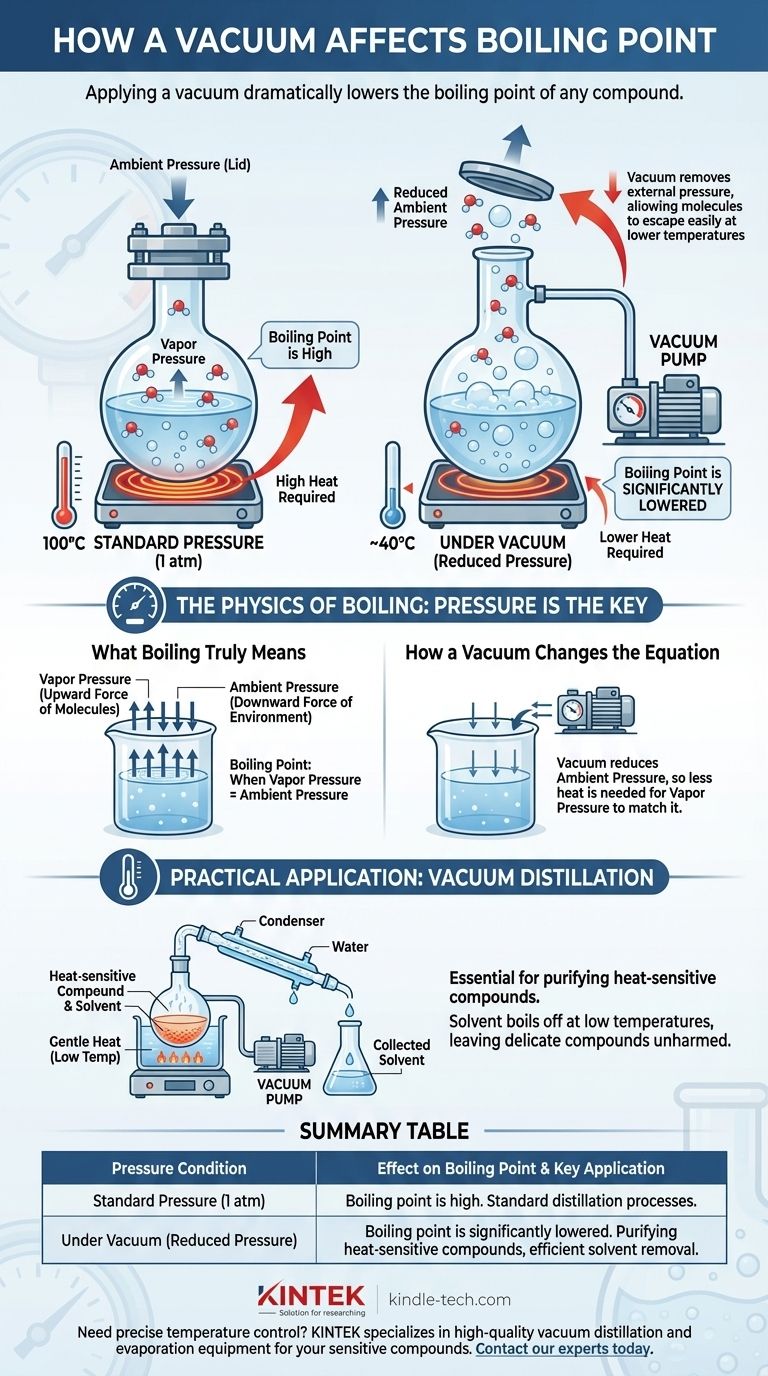
In short, applying a vacuum dramatically lowers the boiling point of any compound. Boiling isn't a fixed temperature but rather the point where a liquid's internal vapor pressure equals the external pressure pushing down on its surface. By creating a vacuum, you remove that external pressure, making it far easier for molecules to escape the liquid phase and turn into a gas at a much lower temperature.
The boiling point of a liquid is not a constant; it is a direct function of the surrounding pressure. Reducing this pressure with a vacuum lowers the energy required for molecules to transition into a gas, causing the substance to boil at a significantly reduced temperature.

The Physics of Boiling: Pressure is the Key
To understand why a vacuum has such a powerful effect, we must first redefine what "boiling" actually is. It's less about a specific temperature and more about a battle of pressures.
What Boiling Truly Means
Boiling is the specific physical state where a liquid's vapor pressure becomes equal to the ambient pressure of its environment.
At this equilibrium point, bubbles of vapor can form within the bulk of the liquid and rise to the surface. This is fundamentally different from simple evaporation, which only occurs at the liquid's surface.
The Role of Vapor Pressure
Every liquid exerts a certain vapor pressure, which is the inherent force of its molecules trying to escape into the gaseous phase.
As you heat a liquid, its molecules gain kinetic energy. This increased energy causes them to push harder against their surroundings, and thus, the vapor pressure increases with temperature.
How Ambient Pressure Acts as a "Lid"
The atmosphere around us exerts a constant pressure on everything, including the surface of liquids. You can think of this ambient pressure as a physical "lid" holding the liquid's molecules in place.
For a liquid to boil, its vapor pressure must become strong enough to "push the lid off." At sea level (1 atmosphere of pressure), water must reach 100°C (212°F) to generate enough vapor pressure to accomplish this.
How a Vacuum Changes the Equation
A vacuum pump works by removing gas molecules from a sealed system, thereby drastically reducing the ambient pressure. This fundamentally alters the conditions required for boiling.
Removing the "Lid"
Applying a vacuum is the equivalent of lifting that pressure "lid." With fewer gas molecules pushing down on the liquid's surface, the molecules can escape much more easily.
This is the same reason water boils at a lower temperature at high altitudes. The atmospheric pressure is lower on a mountain, so less heat is required for the water's vapor pressure to match it.
The New, Lower Boiling Point
Because the external pressure is now much lower, the liquid doesn't need as much heat energy to build its vapor pressure to the boiling point.
The result is that the substance will boil at a temperature far below its standard boiling point. For example, under a strong enough vacuum, water can be made to boil at room temperature.
Practical Application: Vacuum Distillation
This principle is essential in chemistry for purifying heat-sensitive compounds. A chemist can place a mixture under vacuum and gently heat it.
A volatile solvent will boil off at a very low temperature, leaving behind the desired, more delicate compound, which is never exposed to potentially damaging high heat.
Understanding the Trade-offs and Pitfalls
While powerful, using a vacuum to lower the boiling point is not without its challenges and requires careful consideration.
Risk of "Bumping"
Under vacuum, liquids can boil explosively in a phenomenon called bumping. Uneven heating can cause a portion of the liquid to become superheated, suddenly flashing into vapor with violent force. This is typically managed by using boiling chips or constant stirring.
Difficulty with Low-Volatility Substances
For substances with very weak intermolecular forces and thus very low vapor pressures (like oils or ionic liquids), even a high vacuum may not lower the boiling point enough to prevent thermal decomposition. There is a practical limit to the technique's utility.
Equipment and Seal Integrity
Achieving and maintaining a deep vacuum requires specialized pumps and perfectly sealed glassware or containers. Even a minor leak can compromise the system's pressure, causing the boiling point to rise unexpectedly.
Making the Right Choice for Your Goal
Manipulating pressure is a tool, and its application depends entirely on your objective.
- If your primary focus is purifying a heat-sensitive compound: Use vacuum distillation to separate components at temperatures that prevent decomposition or unwanted side reactions.
- If your primary focus is efficiently removing a solvent: Apply a vacuum, often with rotation (as in a rotary evaporator), to rapidly evaporate solvents without requiring high heat.
- If your primary focus is dehydrating a delicate material: Use a deep vacuum to lower the boiling/sublimation point of water, allowing for dehydration at low temperatures (freeze-drying) that preserves the material's structure.
Ultimately, understanding the relationship between pressure and temperature gives you precise control over a substance's physical state.
Summary Table:
| Pressure Condition | Effect on Boiling Point | Key Application |
|---|---|---|
| Standard Pressure (1 atm) | Boiling point is at its standard, high temperature. | Standard distillation processes. |
| Under Vacuum (Reduced Pressure) | Boiling point is significantly lowered. | Purifying heat-sensitive compounds, efficient solvent removal. |
Need precise temperature control for your sensitive compounds? KINTEK specializes in high-quality vacuum distillation and evaporation equipment, including rotary evaporators, designed to protect your delicate materials by lowering boiling points. Our lab equipment ensures efficient, safe processing for researchers and laboratory professionals. Contact our experts today to find the perfect vacuum solution for your application!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Laboratory Rotary Vane Vacuum Pump for Lab Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Electric Heated Hydraulic Vacuum Heat Press for Lab
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
People Also Ask
- Which type of brazing is done in a vacuum? Achieve Clean, Strong Joints with Vacuum Brazing
- What is var material? The Ultimate Guide to High-Purity Vacuum Arc Remelting
- What kind of energy does pyrolysis generate? Converting Waste into Valuable Fuels
- At what temperature is full annealing accomplished by heating? Achieve Maximum Softness for Your Steel
- Why is a laboratory vacuum oven used for the low-temperature drying of acid-functionalized nanoparticles?
- What are the byproducts of pyrolysis oil? Unlocking the Value of Biochar and Syngas
- What is the difference between vacuum cast and vacuum form? Choose the Right Process for Your Prototype
- Why are high-temperature testing furnaces over 2000°C needed for SiC cladding? Validate Gen IV Nuclear Safety



















