In modern medicine, radioactive isotopes are indispensable tools used for two primary purposes: diagnosing and treating disease. By attaching these isotopes to specific molecules, they can function as highly sensitive tracers to illuminate biological processes through imaging or as microscopic weapons to destroy targeted cells, particularly in cancer therapy.
The core principle is straightforward: radioactive isotopes allow physicians to see how organs are functioning and to deliver cell-destroying radiation with high precision, often eliminating the need for more invasive procedures. This is all accomplished by harnessing the predictable energy released during radioactive decay.
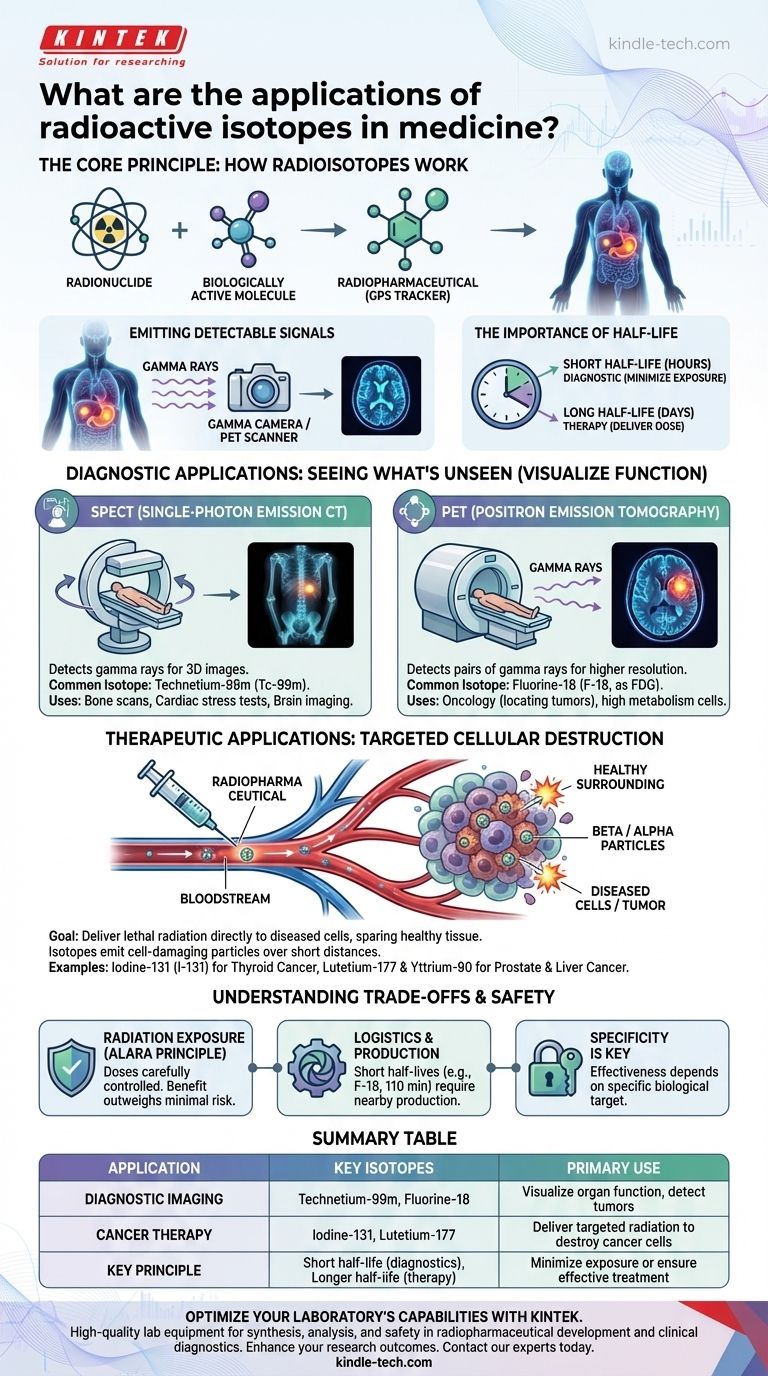
The Core Principle: How Radioisotopes Work in the Body
Functioning as Biological Tracers
A radioactive isotope, or radionuclide, is chemically bonded to a biologically active molecule, creating a radiopharmaceutical.
This compound is designed to be absorbed by a specific organ or tissue. It essentially acts as a GPS tracker, allowing doctors to follow a biological process from outside the body.
Emitting Detectable Signals
As the radionuclide decays, it releases energy in the form of radiation. For diagnostic imaging, the most useful type is gamma rays.
These high-energy photons can pass out of the body and be detected by specialized equipment, such as a gamma camera, to create a detailed image of metabolic activity.
The Importance of Half-Life
An isotope's half-life—the time it takes for half of its radioactive atoms to decay—is a critical factor in its selection.
For diagnostic procedures, isotopes with short half-lives (a few hours) are preferred to minimize the patient's radiation exposure. For therapy, a longer half-life (several days) may be needed to deliver a sufficient dose over time.
Diagnostic Applications: Seeing What's Unseen
The primary value of nuclear medicine imaging is its ability to visualize physiological function, not just anatomical structure like an X-ray or CT scan. It shows how well an organ or system is working.
Single-Photon Emission Computed Tomography (SPECT)
SPECT scans create 3D images by detecting gamma rays from a tracer injected into the patient.
The most common isotope used is Technetium-99m (Tc-99m). Its versatility and ideal half-life (6 hours) make it the workhorse for bone scans, cardiac stress tests, and brain imaging.
Positron Emission Tomography (PET)
PET scans offer higher-resolution images and are particularly valuable in oncology. They detect pairs of gamma rays produced when a positron-emitting radionuclide decays.
The standard for PET is Fluorine-18 (F-18), which is attached to glucose to form FDG. Since cancer cells have a high metabolism and consume more glucose, they light up brightly on a PET scan, revealing the location of tumors.
Therapeutic Applications: Targeted Cellular Destruction
The goal of radionuclide therapy is to deliver a lethal dose of radiation directly to diseased cells while sparing surrounding healthy tissue. This is achieved by using isotopes that emit cell-damaging particles.
The Power of Targeted Delivery
Unlike external beam radiation, radiopharmaceuticals are administered systemically (e.g., by injection) and use the body's own metabolic pathways to concentrate at the target site.
A classic example is Iodine-131 (I-131) for treating thyroid cancer. The thyroid gland naturally absorbs iodine, so it delivers the destructive radiation precisely where it is needed.
Choosing the Right Radiation
Therapeutic isotopes primarily emit beta particles or alpha particles. These particles deposit a large amount of energy over a very short distance.
This characteristic is ideal for therapy, as it destroys the target cell without traveling far enough to damage neighboring healthy cells. Isotopes like Lutetium-177 (for prostate cancer) and Yttrium-90 (for liver cancer) are prominent examples.
Understanding the Trade-offs and Safety
Radiation Exposure
The primary concern with any nuclear medicine procedure is radiation exposure. However, the doses used for diagnostic imaging are carefully controlled and kept As Low As Reasonably Achievable (ALARA).
For a typical diagnostic scan, the radiation dose is comparable to the natural background radiation a person receives over a few years, and the clinical benefit is considered to far outweigh the minimal risk.
Isotope Production and Logistics
Many medically useful isotopes have extremely short half-lives. Fluorine-18, for example, has a half-life of only 110 minutes.
This necessitates a complex logistical chain, often requiring a particle accelerator called a cyclotron to be located near the hospital to produce the isotope just in time for the patient's procedure.
Specificity is Key
Radiopharmaceuticals are not a one-size-fits-all solution. Their effectiveness depends entirely on the presence of a specific biological target. If a tumor does not absorb the tracer molecule, the imaging or therapy will not work.
Matching the Isotope to the Medical Goal
Your clinical objective dictates the choice of radionuclide and its application.
- If your primary focus is high-resolution functional imaging for oncology: PET scans using positron emitters like Fluorine-18 provide unparalleled detail on metabolic activity.
- If your primary focus is versatile, routine diagnostics like bone or cardiac scans: SPECT scans with the gamma emitter Technetium-99m are the established and cost-effective standard.
- If your primary focus is treating a specific cancer with a known biological target: Radionuclide therapy using beta emitters like Iodine-131 or Lutetium-177 delivers targeted radiation.
By selecting the right isotope, medicine can diagnose and treat disease with a level of precision that was once unimaginable.
Summary Table:
| Application | Key Isotopes | Primary Use |
|---|---|---|
| Diagnostic Imaging | Technetium-99m, Fluorine-18 | Visualize organ function, detect tumors |
| Cancer Therapy | Iodine-131, Lutetium-177 | Deliver targeted radiation to destroy cancer cells |
| Key Principle | Short half-life (diagnostics), Longer half-life (therapy) | Minimize exposure or ensure effective treatment |
Optimize Your Laboratory's Capabilities with KINTEK
Are you involved in medical research, radiopharmaceutical development, or clinical diagnostics? The precise application of isotopes like Technetium-99m and Fluorine-18 relies on high-quality lab equipment for synthesis, analysis, and safety.
KINTEK specializes in providing reliable laboratory equipment and consumables that support the entire nuclear medicine workflow. From precise temperature control for synthesis to safe handling and storage solutions, our products help ensure accuracy, efficiency, and safety in your critical work.
Let us help you enhance your research or diagnostic outcomes. Contact our experts today to discuss your specific laboratory needs and discover how KINTEK can be your trusted partner in advancing medical science.
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Electrolytic Electrochemical Cell for Coating Evaluation
- Custom PTFE Teflon Parts Manufacturer for PTFE Ball Valve Seat
- Lab Vibration Mill
- Laboratory High Throughput Tissue Grinding Mill Grinder
People Also Ask
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What is the impact factor of powder metallurgy progress? A 2022 Analysis & Context
- What are the advantages and disadvantages of sieve analysis test? A Guide to Effective Particle Sizing
- What are alloys in simple words? Unlock the Power of Engineered Materials
- What material is a PTFE cleaning basket made of? Unlocking Superior Chemical and Thermal Resistance



















