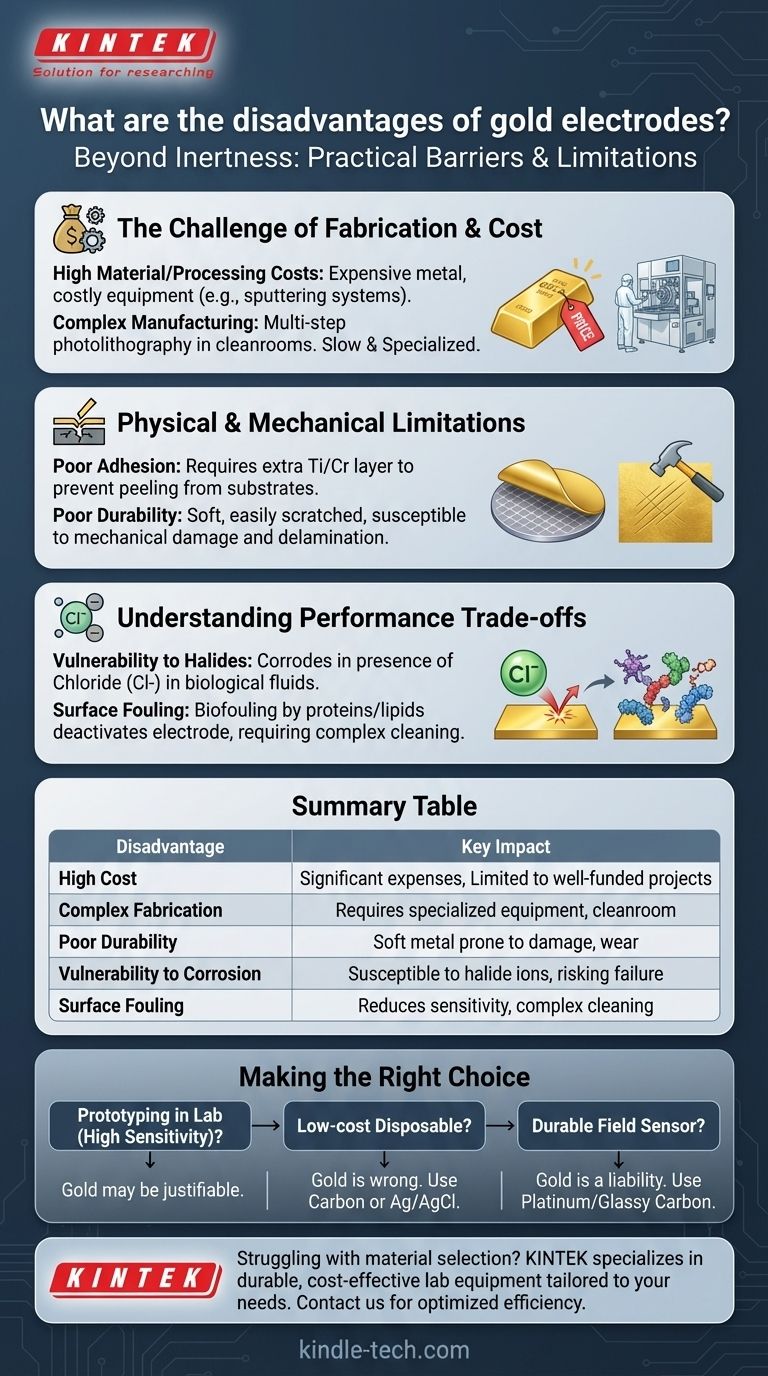
Beyond its reputation for inertness, the primary disadvantages of using gold for electrodes are its high cost and the complex, specialized fabrication processes it requires. These practical barriers often make it an impractical choice for applications outside of well-funded research or high-margin medical devices, limiting its widespread adoption.
The core issue with gold electrodes is not a fundamental failure in performance, but a series of practical and physical limitations. The high cost, manufacturing complexity, and poor mechanical durability often outweigh its benefits in many real-world applications.

The Challenge of Fabrication and Cost
While gold is an excellent conductor and biocompatible, the journey from raw material to a functional electrode is fraught with practical and financial hurdles.
High Material and Processing Costs
Gold is an expensive precious metal, making the raw material cost significant, especially for larger surfaces or mass production.
Furthermore, the equipment needed for fabrication—such as thermal evaporators, sputtering systems, and photolithography tools—represents a substantial capital investment.
Complex Manufacturing Processes
Creating thin-film gold electrodes typically requires a multi-step process known as photolithography, which must be performed in a highly controlled cleanroom environment.
This reliance on specialized facilities and trained personnel makes fabrication slow, expensive, and inaccessible to many organizations.
The Critical Adhesion Problem
Gold exhibits very poor adhesion to common substrates like silicon, glass, or flexible polymers.
To prevent the gold layer from peeling off, an intermediate adhesion layer, typically made of titanium or chromium, must be deposited first. This adds an extra step to the manufacturing process and another potential point of failure.
Physical and Mechanical Limitations
Gold's value as a precious metal comes from its chemical stability, but its physical properties present significant challenges for electrode durability.
Poor Mechanical Durability
Gold is an extremely soft metal, making it highly susceptible to scratches and mechanical damage from physical contact, cleaning, or abrasion.
This lack of robustness makes it unsuitable for applications requiring long-term use, repeated cleaning, or any form of physical wear and tear.
Susceptibility to Delamination
Even with an adhesion layer, the bond between the gold and the substrate can be a weak point. Thermal stress, flexing, or chemical exposure can cause the gold film to delaminate or peel away, leading to device failure.
Understanding the Trade-offs in Performance
While often considered the "gold standard" for its chemical inertness, gold is not without its electrochemical and operational weaknesses.
Vulnerability to Halides
Despite its general stability, gold is not entirely inert. It can be oxidized and corroded by halide ions, particularly chloride (Cl-), which is ubiquitous in biological fluids like blood plasma and urine.
This can lead to signal drift or complete failure of a sensor in its intended environment.
Surface Fouling
Like most electrode surfaces, gold is prone to biofouling, where molecules like proteins, lipids, and other biomolecules nonspecifically adsorb onto the surface.
This deactivates the electrode, reducing its sensitivity and requiring either disposal or complex cleaning and regeneration procedures that can damage the soft surface.
Making the Right Choice for Your Application
Selecting an electrode material requires balancing ideal performance with practical constraints like cost, scalability, and durability.
- If your primary focus is prototyping with the highest possible sensitivity in a controlled lab: Gold may be a justifiable choice, as performance is prioritized over cost and durability.
- If your primary focus is a low-cost, mass-produced disposable device: Gold is almost certainly the wrong material. Screen-printed carbon or silver/silver chloride (Ag/AgCl) are far more economical and scalable.
- If your primary focus is a durable, reusable sensor for field deployment: Gold's softness and adhesion issues are major liabilities. Consider more robust materials like platinum or glassy carbon.
Understanding these practical limitations allows you to choose an electrode material based on the specific, real-world demands of your project, not just on chemical reputation.
Summary Table:
| Disadvantage | Key Impact |
|---|---|
| High Cost | Significant material and processing expenses, limiting use to well-funded projects. |
| Complex Fabrication | Requires specialized equipment (e.g., sputtering systems) and cleanroom facilities. |
| Poor Durability | Soft metal prone to scratches, delamination, and damage from wear and tear. |
| Vulnerability to Corrosion | Susceptible to halide ions (e.g., chloride) in biological fluids, risking sensor failure. |
| Surface Fouling | Prone to biofouling, reducing sensitivity and requiring complex cleaning procedures. |
Struggling with electrode material selection for your lab equipment? KINTEK specializes in providing durable, cost-effective lab equipment and consumables tailored to your specific needs—whether you're prototyping, scaling production, or deploying field-ready sensors. Our experts can help you choose the right materials to balance performance, durability, and budget. Contact us today to optimize your lab's efficiency and achieve reliable results!
Visual Guide
Related Products
- Gold Disc Electrode
- Gold Electrochemical Sheet Electrode Gold Electrode
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What criteria are used during the visual inspection of electrodes? Essential Quality Assessment for Your Lab
- What operations should be performed on a titanium electrode before use? Ensure Safety and Maximize Performance
- What are the proper procedures for handling a titanium electrode after use? Ensure Longevity and Peak Performance
- What advantages do BDD electrodes offer in Kolbe electrolysis? Maximize Durability and Electrochemical Efficiency
- What is the role of a high-precision potentiostat in indium electrowinning? Optimize Your Kinetic Studies Today
- What critical role does a platinum counter electrode play in bioelectrochemical reactions? Ensure Data Purity and Stability
- What are the key performance characteristics of a metal disk electrode? Ensuring Accurate Electrochemical Measurements
- How should a titanium electrode be regularly maintained and cleaned? Protect Your Investment and Maximize Performance














