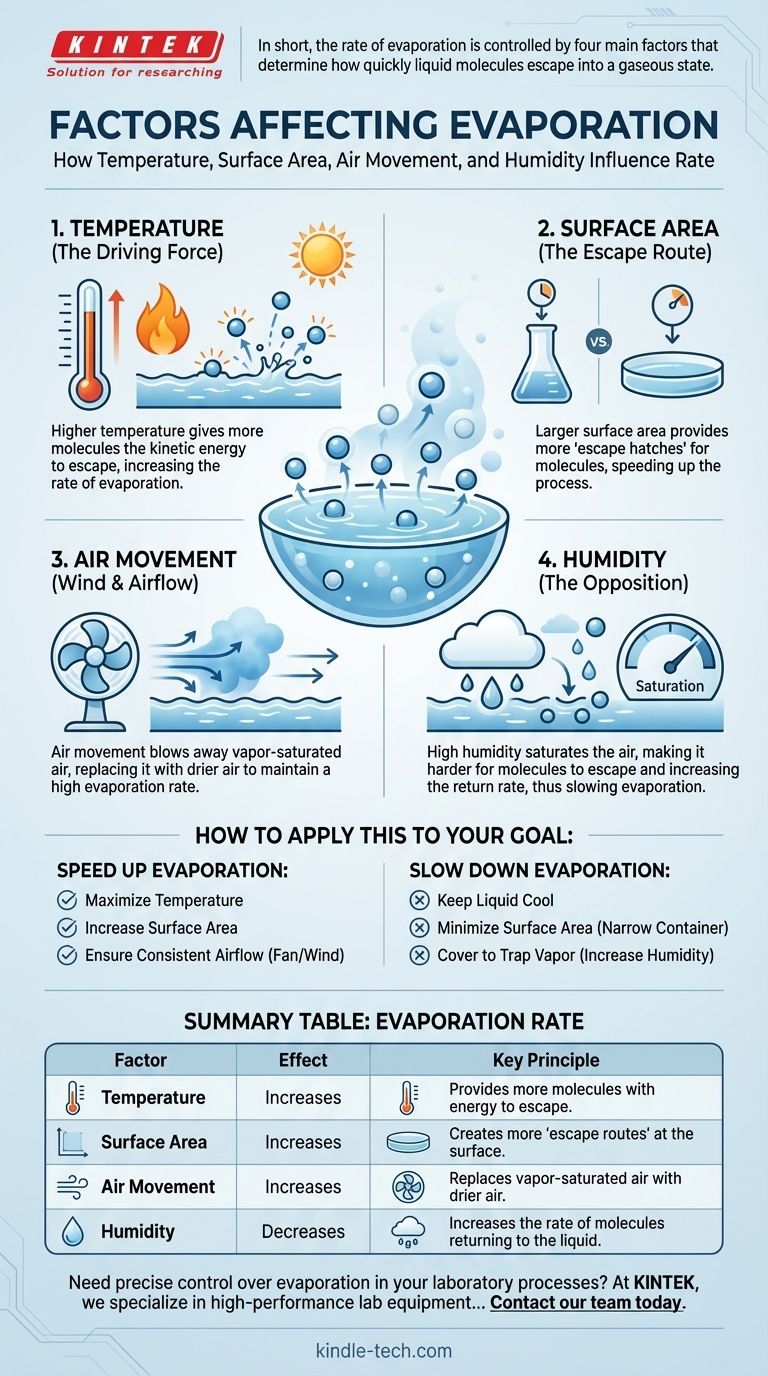
In short, the rate of evaporation is primarily controlled by four factors: temperature, surface area, air movement, and the humidity of the surrounding air. These elements work together to determine how quickly a liquid's molecules can gain enough energy to escape and transition into a gaseous state.
The core principle of evaporation is a battle between molecules escaping a liquid and molecules returning to it. Every influencing factor simply tips this balance, either by making it easier for molecules to leave or harder for them to come back.

The Driving Force: Temperature and Molecular Energy
At its heart, evaporation is about energy. The single factor that provides the most direct energy input is temperature, which makes it the most critical driver of the entire process.
What is Evaporation at the Molecular Level?
Evaporation occurs when molecules within a liquid gain enough kinetic energy to overcome the forces holding them together. Once they break these bonds, they escape from the surface and become a gas or vapor.
The Direct Impact of Temperature
Temperature is a measure of the average kinetic energy of molecules in a substance. When you heat a liquid, you are increasing this average energy.
This means more molecules at any given moment will possess the minimum "escape energy" required to break free from the liquid's surface.
How Temperature Affects the Rate
Because a higher temperature equips more molecules with the energy to escape, it directly and significantly increases the rate of evaporation. A puddle will disappear much faster on a hot day than on a cold one for this simple reason.
The Escape Route: Surface Area and Airflow
While temperature provides the energy for escape, the physical conditions at the liquid's surface determine how easily that escape can happen.
Why a Larger Surface Area is Faster
Only molecules located at the surface of a liquid can evaporate. The more surface area is exposed to the air, the more "escape hatches" are available for high-energy molecules.
Spilling a glass of water creates a wide, thin puddle that evaporates quickly. The same amount of water left in the glass will take much longer to evaporate because its surface area is far smaller.
The Role of Air Movement (Wind)
As a liquid evaporates, the air just above its surface becomes saturated with vapor. This creates a humid micro-environment that makes it harder for more molecules to escape.
Wind or any form of airflow blows this vapor-saturated air away, replacing it with drier air. This maintains a steep concentration gradient, encouraging the liquid to continue evaporating at a high rate.
The Opposition: Humidity in the Air
Evaporation is not a one-way street. While molecules are escaping the liquid, molecules of vapor in the air are also condensing back into it. The net rate of evaporation depends on the balance between these two processes.
How Humidity Acts as a Barrier
Humidity is the amount of water vapor already present in the air. If the air is already highly saturated (high humidity), there is less "room" for new water molecules.
This high concentration of vapor in the air increases the rate at which molecules return to the liquid. When the rate of return equals the rate of escape, net evaporation stops.
The Interplay of Factors
This is why a hot but humid day can feel so uncomfortable and prevent sweat from evaporating effectively. Even with high temperatures providing energy, the saturated air suppresses the net evaporation rate. The most rapid evaporation occurs on hot, dry, and windy days.
How to Apply This to Your Goal
Understanding these factors allows you to predict and control the rate of evaporation for any practical purpose.
- If your primary focus is to speed up evaporation: Maximize temperature, spread the liquid out to increase surface area, and use a fan or wind to ensure consistent airflow.
- If your primary focus is to slow down evaporation: Keep the liquid cool, store it in a container with a narrow opening to minimize surface area, and cover it to trap vapor and increase local humidity.
Ultimately, mastering these principles gives you direct control over this fundamental physical process.
Summary Table:
| Factor | Effect on Evaporation Rate | Key Principle |
|---|---|---|
| Temperature | Increases | Provides more molecules with the energy to escape. |
| Surface Area | Increases | Creates more 'escape routes' for molecules at the surface. |
| Air Movement | Increases | Replaces vapor-saturated air with drier air. |
| Humidity | Decreases | Increases the rate of molecules returning to the liquid. |
Need precise control over evaporation in your laboratory processes? At KINTEK, we specialize in high-performance lab equipment, including ovens, incubators, and environmental chambers that provide the exact temperature and airflow conditions you need to master evaporation for research, sample preparation, and chemical synthesis. Let our experts help you select the right equipment to optimize your workflow. Contact our team today for a personalized consultation!
Visual Guide
Related Products
- Benchtop Laboratory Freeze Dryer for Lab Use
- Benchtop Laboratory Vacuum Freeze Dryer
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What is the source of vacuum evaporation? Energy and Vacuum for High-Purity Coatings
- What is thermal effect via evaporation? A Simple Guide to Thin-Film Deposition
- What is the thermal evaporation of gold? A Simple Guide to Gold Thin Film Deposition
- What are the disadvantages of e-beam evaporation? High Costs and Geometric Limitations Explained
- What type of deposition is resulted at high vacuum? Achieve Pure, High-Performance Thin Films with PVD
- What is the thermal evaporation method in thin film? A Guide to Simple, Cost-Effective PVD
- What is the vaporization of zinc? A Critical Safety & Quality Challenge in Welding
- What are the advantages of electron beam physical vapor deposition? Achieve High-Purity, High-Speed Thin Films



















