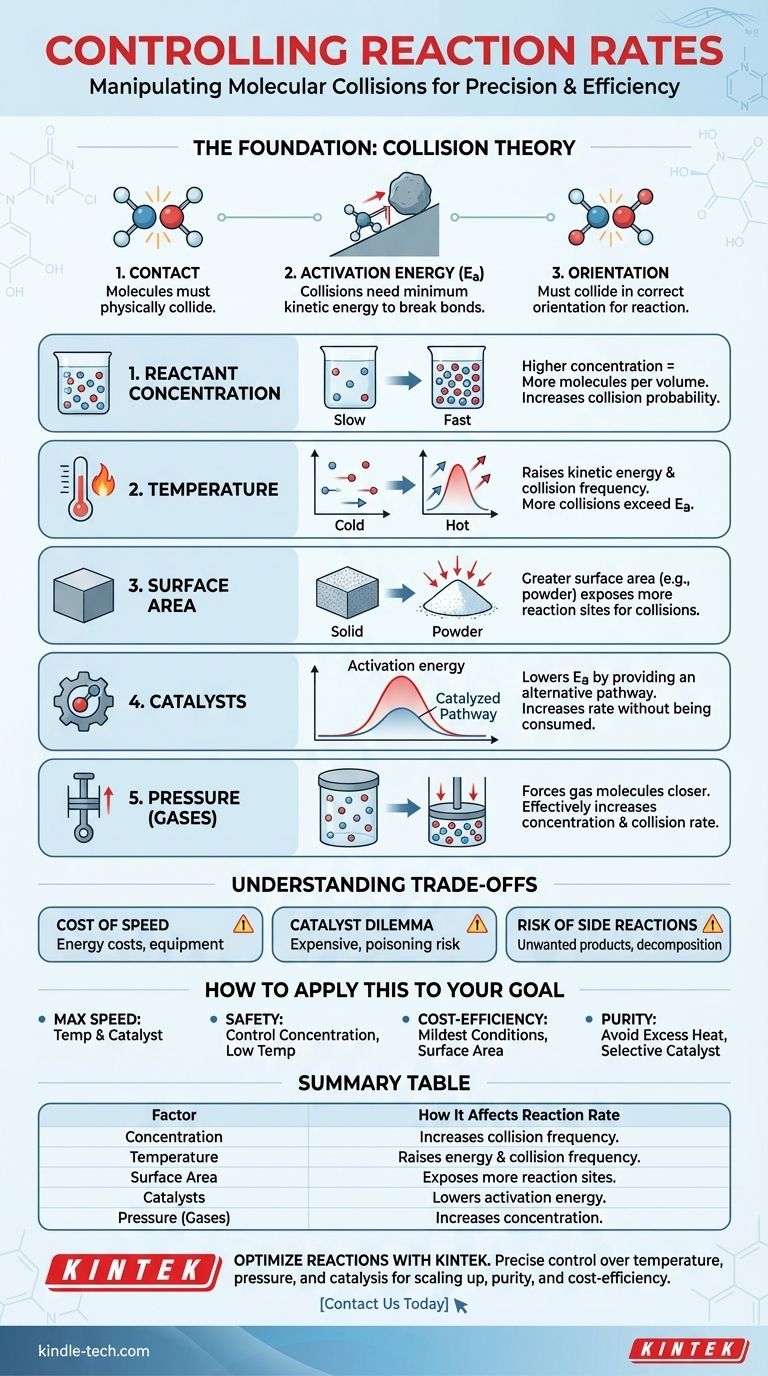
In essence, five primary factors control the rate of a chemical reaction: the concentration of reactants, temperature, the physical state and surface area of the reactants, the presence of a catalyst, and for gases, pressure. Each of these factors influences the frequency and energy of molecular collisions, which is the fundamental driver of all chemical change.
The speed of any chemical reaction is determined by how often reactant molecules collide with the correct orientation and with enough energy to overcome the reaction's activation energy barrier. Everything that controls reaction rates is simply a lever to manipulate the frequency and effectiveness of these collisions.

The Foundation: Collision Theory
To control a reaction, you must first understand that reactions are not instantaneous events. They are the result of physical interactions between molecules. This is explained by Collision Theory.
The Need for Contact
For a reaction to occur, reactant molecules must physically collide with one another. If they are in separate containers, no reaction can happen. The rate of the reaction is directly tied to the rate of these collisions.
The Energy Requirement (Activation Energy)
A collision alone is not enough. The colliding molecules must possess a certain minimum amount of kinetic energy, known as the activation energy (Ea). This energy is required to break existing chemical bonds so that new ones can form.
Think of activation energy as a hill you must push a boulder over. A gentle nudge (a low-energy collision) won't do anything. You need to provide enough of a push (sufficient energy) to get it to the top of the hill, after which it can roll down the other side and release energy.
The Orientation Factor
Finally, molecules must collide in a specific orientation that allows the correct bonds to break and new ones to form. A random collision at the wrong angle, even if it's energetic enough, will not result in a reaction.
The Five Levers for Controlling Reaction Rate
Understanding collision theory gives you five distinct levers to pull to speed up or slow down a reaction. Each one works by influencing collision frequency, collision energy, or the activation energy barrier itself.
1. Reactant Concentration
Increasing the concentration of reactants means there are more molecules packed into a given volume. This directly increases the probability of them colliding, thus increasing the reaction rate.
2. Temperature
Raising the temperature has a powerful, two-fold effect. First, it increases the kinetic energy of molecules, making them move faster and collide more frequently. Second, and more importantly, it increases the energy of these collisions, meaning a higher percentage of them will have enough energy to overcome the activation energy barrier.
3. Physical State and Surface Area
This is most relevant for reactions involving different phases, like a solid reacting with a liquid (heterogeneous reactions). A solid block has a limited surface area where collisions can occur. Grinding that same block into a fine powder dramatically increases its surface area, exposing more molecules and creating far more sites for reactions to happen.
4. Catalysts
A catalyst increases the reaction rate without being consumed in the process. It does this by providing an alternative reaction pathway with a lower activation energy.
Returning to our analogy, a catalyst is like building a tunnel through the hill. You no longer need to push the boulder all the way to the top; you can get it to the other side with much less effort. This allows more molecules to react successfully, even at lower temperatures.
5. Pressure (for Gaseous Reactions)
For reactions involving gases, increasing the pressure forces the gas molecules closer together. This is effectively the same as increasing their concentration, leading to more frequent collisions and a faster reaction rate.
Understanding the Trade-offs
Manipulating reaction rates is not without consequences. An effective technical advisor must consider the practical and economic implications of each choice.
The Cost of Speed
Increasing temperature and pressure requires energy, which costs money. It also may demand specialized, high-pressure reactors that are expensive to build and maintain, introducing safety considerations.
The Catalyst Dilemma
Catalysts can be highly effective but are often expensive (e.g., using precious metals like platinum or palladium). They can also be very specific to one reaction and can be rendered inactive by impurities, a process known as "catalyst poisoning."
Risk of Unwanted Side Reactions
Pushing a reaction too hard, especially with excessive heat, can lead to undesirable outcomes. Reactants or products might begin to decompose, or the increased energy can enable unwanted side reactions, reducing the purity and yield of your desired product.
How to Apply This to Your Goal
Your strategy for controlling a reaction depends entirely on what you are trying to achieve.
- If your primary focus is maximum speed: The most powerful levers are increasing temperature and using an effective catalyst, as both dramatically increase the number of successful, energetic collisions.
- If your primary focus is safety and control: Carefully managing reactant concentration (e.g., by adding one reactant slowly to another) and using the lowest effective temperature are the best approaches.
- If your primary focus is cost-efficiency: Optimize for the mildest conditions possible. This may involve finding an affordable catalyst or increasing surface area to avoid the high energy costs of extreme heat and pressure.
- If your primary focus is product purity: Avoid excessively high temperatures that could cause decomposition or side reactions, and ensure your catalyst is highly selective for the desired reaction.
Ultimately, controlling a chemical reaction is the art of precisely managing the conditions that govern molecular collisions.
Summary Table:
| Factor | How It Affects Reaction Rate |
|---|---|
| Concentration | Higher concentration increases collision frequency between reactant molecules. |
| Temperature | Raises molecular energy and collision frequency; more collisions exceed activation energy. |
| Surface Area | Greater surface area (e.g., powdered solid) exposes more reaction sites for collisions. |
| Catalysts | Lowers activation energy by providing an alternative reaction pathway. |
| Pressure (Gases) | Increases gas molecule concentration, leading to more frequent collisions. |
Optimize your chemical reactions with precision equipment from KINTEK. Whether you're scaling up production, ensuring product purity, or maximizing cost-efficiency, our lab equipment and consumables are designed to give you precise control over reaction conditions. Let our experts help you select the right tools to manage temperature, pressure, and catalysis for your specific needs. Contact us today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press 25T 30T 50T
People Also Ask
- What are the requirements for reaction vessels for alkaline PHA recovery? Ensure High Purity and Material Integrity
- What function does a high-pressure reactor serve in magnetic wood synthesis? Expert Guide to In-Situ Mineralization
- What are the individual parts of a bioreactor? Unlock the Key Components for Optimal Cell Growth
- What are the design advantages of SHS reactors? Streamline Production with Compact, High-Efficiency Systems
- Why use a stirred high-pressure autoclave for plastic pyrolysis? Maximize Yields with Advanced Mass Transfer
- Why is a glass reactor under nitrogen protection used for PDMS-b-PCL synthesis? Ensure Purity & Precise Polymerization
- What is the role of a heating reactor with a vacuum system in cable material preparation? Ensure Flawless Insulation
- What is a fixed bed pyrolysis reactor? A Simple, Cost-Effective Solution for Biochar Production



