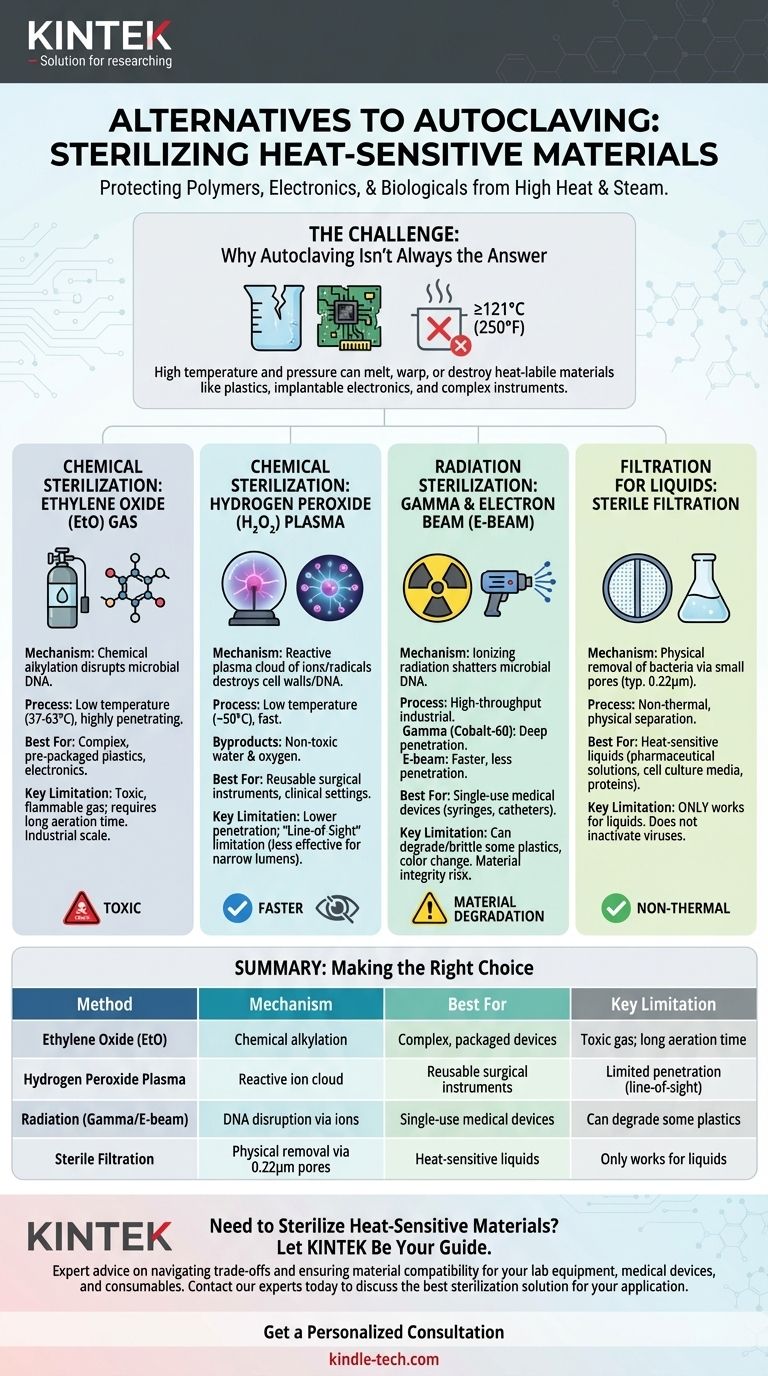
For heat-sensitive materials, the primary alternatives to steam autoclaving are chemical sterilization using ethylene oxide (EtO) or hydrogen peroxide plasma, and radiation sterilization using gamma or electron beam (E-beam) methods. Each technique leverages a different mechanism to achieve sterility without the high temperatures that can damage polymers, electronics, or biologicals.
The core challenge is not just finding an alternative to heat, but matching the right sterilization method to your specific material, budget, and safety requirements. Moving away from steam introduces a new set of trade-offs involving chemical toxicity, material degradation, and process complexity.
Why Autoclaving Isn't Always the Answer
While autoclaving is a gold standard for its reliability and non-toxic nature, its reliance on high-pressure steam makes it fundamentally unsuitable for a wide range of modern materials.
The Impact of High Heat and Steam
An autoclave operates at temperatures typically at or above 121°C (250°F). This intense heat, combined with pressurized steam, will melt, warp, or destroy many common polymers and plastics. Furthermore, the high moisture content can damage sensitive electronics or paper-based products.
Common Heat-Sensitive Materials
Materials that cannot withstand autoclaving are often referred to as heat-labile. This category includes many single-use medical devices, certain plastics like polyethylene and PVC, implantable electronics, and complex surgical instruments with delicate components.
Key Alternatives to Heat Sterilization
When heat is not an option, sterilization relies on either chemical reactions or high-energy radiation to inactivate microorganisms.
Chemical Sterilization: Ethylene Oxide (EtO) Gas
Ethylene oxide is a highly effective alkylating agent that disrupts the DNA of microorganisms, preventing them from reproducing. It is a gas that can penetrate and sterilize large volumes of complex items, even when they are already packaged.
Because EtO sterilization occurs at much lower temperatures (typically 37-63°C), it is a long-standing industry standard for sterilizing plastics, electronics, and other heat-sensitive devices.
Chemical Sterilization: Hydrogen Peroxide (H₂O₂) Plasma
This method involves vaporizing a hydrogen peroxide solution in a low-pressure chamber to create a reactive plasma cloud. This cloud of ions and radicals rapidly sterilizes materials by destroying microbial cell walls and DNA.
The process operates at a low temperature (around 50°C) and is significantly faster than EtO. Its primary byproducts are non-toxic water and oxygen, making it a safer alternative for healthcare settings.
Radiation Sterilization: Gamma & Electron Beam (E-beam)
This is a high-throughput industrial process used for many pre-packaged, single-use medical products. Materials are exposed to a controlled dose of ionizing radiation, which shatters the DNA of any contaminating microbes.
Gamma radiation uses a radioisotope source (Cobalt-60) and has exceptional penetration, making it ideal for dense products on pallets. E-beam sterilization uses a stream of high-energy electrons, offering faster processing times but with less penetration than gamma rays.
Filtration for Liquids
For heat-sensitive liquids, such as pharmaceutical solutions, protein formulations, or cell culture media, the only viable method is sterile filtration. The liquid is passed through a filter with a pore size small enough (typically 0.22 microns) to physically block and remove all bacteria.
Understanding the Trade-offs and Risks
Choosing an alternative to autoclaving means accepting a different set of limitations. There is no single perfect solution.
Ethylene Oxide (EtO): The Power and the Poison
While highly effective and compatible with most materials, EtO is a toxic, flammable, and carcinogenic gas. Items sterilized with EtO require a lengthy aeration period to remove residual gas, significantly increasing total processing time and posing a risk to operators if not handled properly.
Radiation: The Risk of Material Degradation
Ionizing radiation can alter the physical properties of certain polymers. Some plastics can become brittle or change color after being irradiated, a critical factor for medical implants or device components where material integrity is paramount.
Chemical Plasma: The Line-of-Sight Limitation
Hydrogen peroxide plasma has lower penetration power than EtO gas. It can be less effective for sterilizing devices with very long, narrow lumens or complex internal geometries where the plasma cannot reach all surfaces.
Cost and Accessibility
EtO and radiation sterilization are large-scale industrial processes requiring significant capital investment and specialized facilities. While smaller H₂O₂ plasma sterilizers are available for hospitals, they are still far more complex and expensive than a standard autoclave.
Making the Right Choice for Your Material
Your final decision must be guided by your material's composition and your operational needs.
- If your primary focus is single-use medical devices (syringes, catheters): Radiation (gamma or E-beam) is the industry standard due to its high throughput and reliability.
- If your primary focus is complex, heat-sensitive instruments for reuse: Hydrogen peroxide plasma is often the preferred choice in clinical settings for its safety and fast turnaround.
- If your primary focus is sterilizing heat-labile liquids (e.g., cell culture media): Sterile filtration is the only appropriate method to preserve the integrity of the solution.
- If your primary focus is bulk sterilization of diverse packaged goods: Ethylene oxide (EtO) remains a powerful option, provided you can manage the significant safety risks and long processing times.
Ultimately, selecting the correct sterilization method is a critical decision that directly impacts product safety, material integrity, and operational efficiency.

Summary Table:
| Method | Mechanism | Best For | Key Limitation |
|---|---|---|---|
| Ethylene Oxide (EtO) | Chemical alkylation | Complex, packaged devices | Toxic gas; long aeration time |
| Hydrogen Peroxide Plasma | Reactive ion cloud | Reusable surgical instruments | Limited penetration (line-of-sight) |
| Radiation (Gamma/E-beam) | DNA disruption via ions | Single-use medical devices | Can degrade some plastics |
| Sterile Filtration | Physical removal via 0.22μm pores | Heat-sensitive liquids | Only works for liquids |
Need to Sterilize Heat-Sensitive Materials? Let KINTEK Be Your Guide.
Choosing the right sterilization method is critical for the safety and integrity of your lab equipment, medical devices, and consumables. KINTEK specializes in providing solutions and expert advice for all your laboratory needs.
✅ We help you navigate the trade-offs between methods like EtO, plasma, and radiation. ✅ Ensure material compatibility and process efficiency for your specific application.
Contact our experts today to discuss the best sterilization solution for your heat-sensitive materials.
Get a Personalized Consultation
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Graphite Vacuum Continuous Graphitization Furnace
- Laboratory High Throughput Tissue Grinding Mill Grinder
People Also Ask
- How is an autoclave utilized in antimicrobial experiments? Ensure Precise Nanoparticle Research Integrity
- What role do laboratory autoclaves play in pectin extraction? Optimize Prebiotic Yield from Citrus and Apple Biomass
- Why is a laboratory autoclave necessary for Postgate Medium B (PMB)? Ensure Pure SRB Cultures & Accurate MIC Research
- What is the primary function of a laboratory Autoclave in seaweed hydrolysates? Sterilize and Optimize Fermentation
- What is the primary function and principle of autoclaving? Master Lab Sterilization with High-Pressure Steam



















