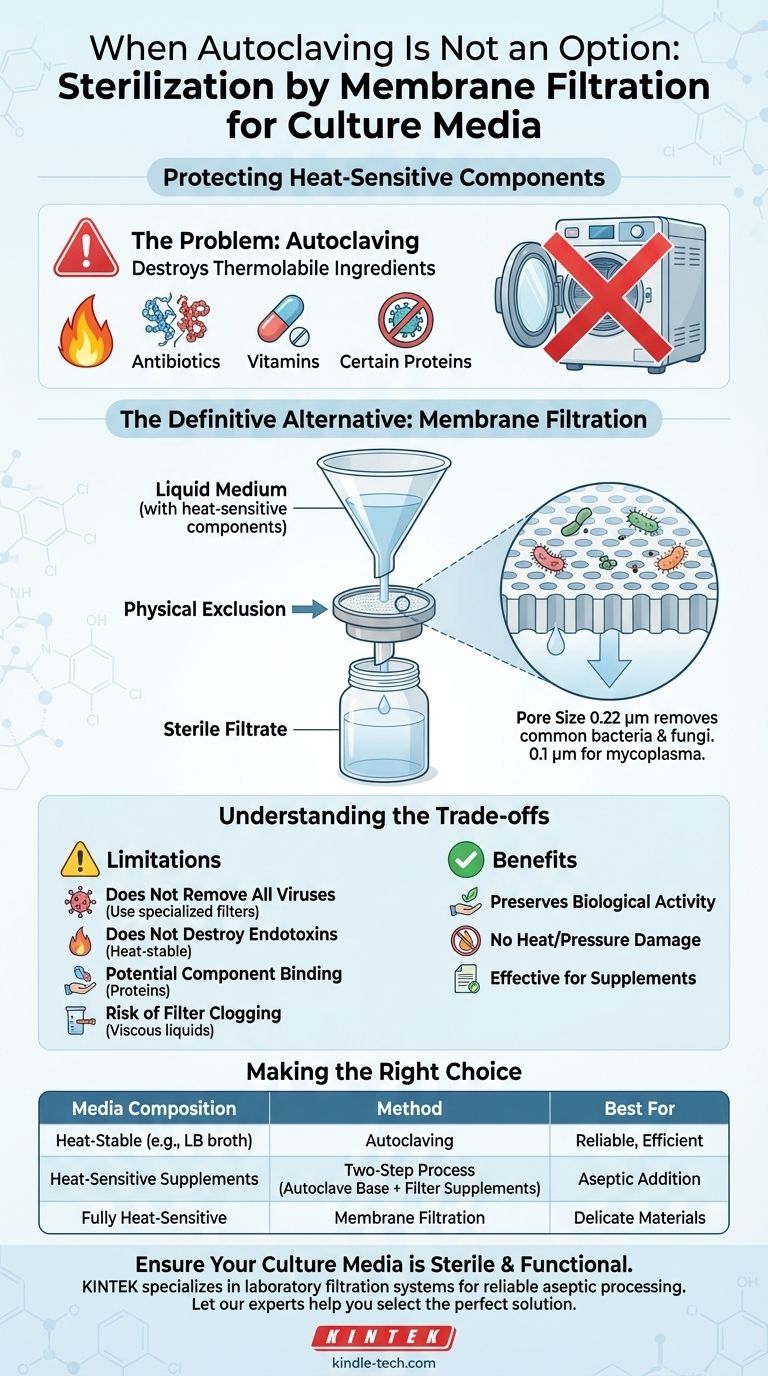
For culture media with heat-sensitive components, the definitive alternative to autoclaving is sterilization by membrane filtration. This physical removal method ensures that vital, thermolabile ingredients like antibiotics, vitamins, or certain proteins are not degraded by the high temperatures and pressures of an autoclave. It effectively removes microorganisms while preserving the medium's intended biological activity.
The core principle is simple: autoclaving kills microbes with heat, which can destroy your media's delicate components. Filtration physically removes microbes by passing the liquid through a microscopic sieve, preserving the integrity of the final solution.

Why Autoclaving Isn't Always the Answer
While autoclaving is the gold standard for sterilizing many materials, it relies on intense heat (typically 121°C) and pressure. This process is destructive by design and is unsuitable for certain chemical compounds.
The Problem of Heat-Sensitive Components
Many specialized culture media require supplements that are thermolabile, meaning they break down or become inactive when exposed to high temperatures.
As noted, substances like urea, certain serums, and some proteins will degrade under autoclave conditions. Other common heat-sensitive additives include most antibiotics, specific vitamins, and various growth factors.
The Risk of Chemical Degradation
Autoclaving these components doesn't just damage them—it can fundamentally alter the properties of your culture medium.
For example, if an antibiotic in a selective medium is destroyed, the medium loses its selective power, allowing unwanted organisms to grow. Similarly, degraded growth factors will fail to support the intended cell cultures.
The Principle of Sterilization by Filtration
Filtration is not a method of killing microorganisms; it is a method of physical exclusion. The process is straightforward but requires meticulous attention to aseptic technique.
How Membrane Filtration Works
The liquid medium is passed through a filter membrane containing pores of a specific, uniform size. Microorganisms, being larger than the pores, are trapped on the surface of the membrane.
The sterile liquid that passes through—the filtrate—is then collected in a pre-sterilized container. This entire procedure must be performed in a sterile environment, such as a laminar flow hood, to prevent re-contamination.
Choosing the Right Pore Size
The effectiveness of filtration depends entirely on using the correct pore size.
The industry standard for sterilizing filtration is a membrane with a 0.22 micrometer (µm) pore size. This is small enough to reliably remove all common bacteria and fungi. For specific applications requiring the removal of smaller microbes like mycoplasma, a 0.1 µm filter may be necessary.
Understanding the Trade-offs and Limitations
While filtration is essential for heat-sensitive liquids, it is not a universal replacement for autoclaving. It has specific limitations that you must understand.
It Does Not Remove All Viruses
Many viruses are smaller than 0.22 µm and can pass through a standard sterilizing filter. If viral contamination is a concern, other methods or specialized filters may be required.
It Does Not Destroy Endotoxins
Bacterial endotoxins are heat-stable molecules released from the cell walls of Gram-negative bacteria. Filtration may remove the bacteria, but any endotoxins already present in the solution will pass through the filter.
Potential for Component Binding
Some molecules, particularly proteins, can adsorb (stick) to the filter membrane. This can lead to a slight reduction in the concentration of that component in the final filtered medium, which may be significant for highly sensitive applications.
Risk of Filter Clogging
Viscous liquids or media containing significant particulate matter can quickly clog the filter membrane, slowing down the process and potentially causing the filter to rupture under pressure.
Making the Right Choice for Your Media
The correct sterilization method depends entirely on the composition of your culture medium. The goal is to achieve sterility while preserving the medium's biological function.
- If your media contains only heat-stable components (e.g., basal media like LB or TSB): Autoclaving remains the most reliable, efficient, and cost-effective method of sterilization.
- If your media requires heat-sensitive supplements (e.g., antibiotics or vitamins): Use a two-step process. Autoclave the heat-stable base medium first, allow it to cool to a safe temperature, and then aseptically add the filter-sterilized supplements.
- If your entire medium is composed of delicate, heat-sensitive materials: The complete, final solution must be sterilized using membrane filtration into a sterile receiving vessel.
Ultimately, choosing the right method is about understanding your components to ensure your final culture medium is both sterile and effective.
Summary Table:
| Sterilization Method | Best For | Key Limitation |
|---|---|---|
| Autoclaving | Heat-stable media (e.g., LB broth) | Degrades thermolabile components |
| Membrane Filtration | Heat-sensitive media (e.g., with antibiotics) | Does not remove viruses or endotoxins |
Ensure your culture media is both sterile and fully functional. If your work relies on heat-sensitive components like antibiotics, vitamins, or proteins, the right sterilization equipment is critical. KINTEK specializes in laboratory filtration systems and consumables designed for reliable, aseptic processing. Let our experts help you select the perfect solution to protect your valuable media. Contact us today to discuss your specific needs!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Pulsating Vacuum Desktop Steam Sterilizer
- Laboratory High Pressure Steam Sterilizer Vertical Autoclave for Lab Department
- Portable Digital Display Automatic Laboratory Sterilizer Lab Autoclave for Sterilization Pressure
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What temperature must be reached for sterilization in 10-12 minutes? Achieve Rapid, Reliable Sterility with Flash Autoclaving
- Do you need to autoclave glassware? A Guide to Sterilization vs. Cleaning
- What is a lab autoclave? Your Guide to Sterilization with Pressurized Steam
- What is the use of autoclave in medical? The Critical Role of Sterilization in Patient Safety
- What are the advantages of autoclaving in hospitals? Achieve Unmatched Sterilization for Patient Safety

















