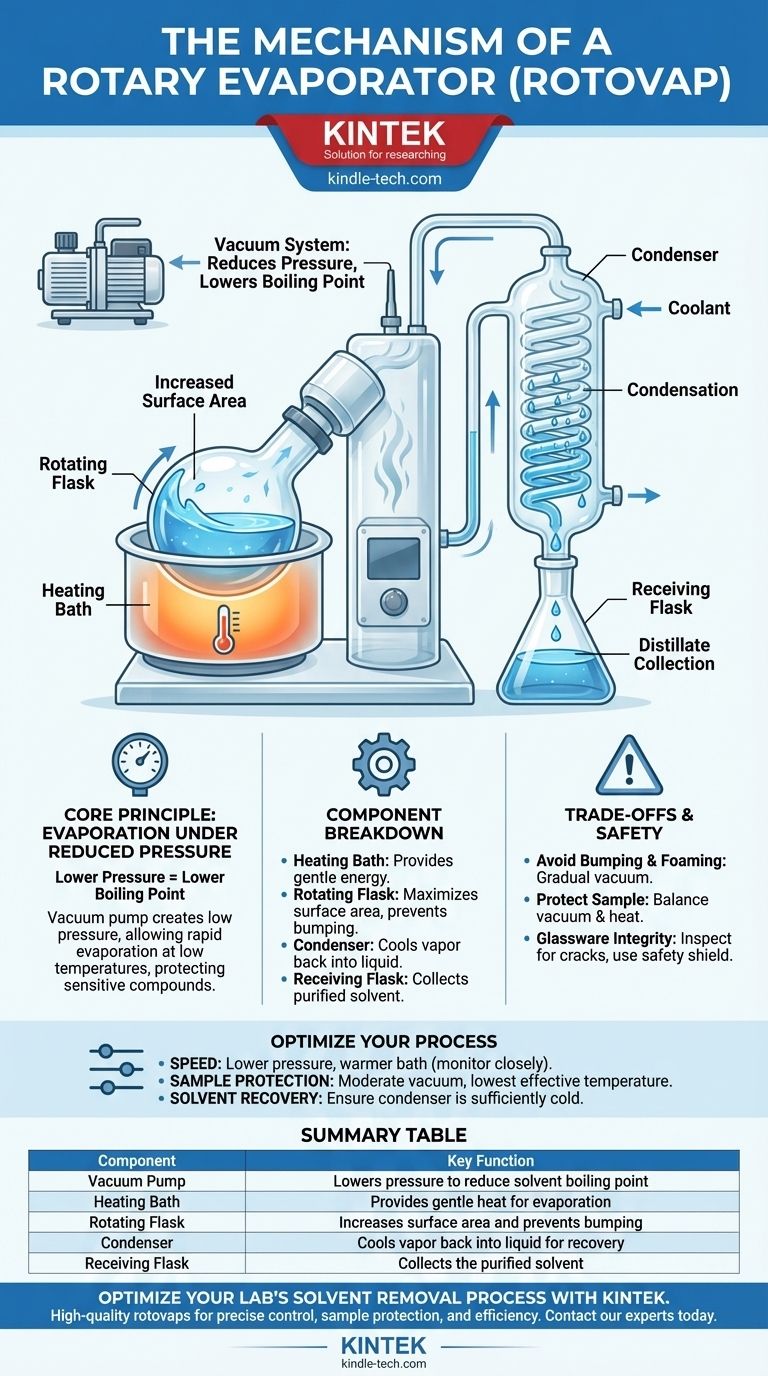
At its core, a rotary evaporator, commonly known as a rotovap, is a device used in chemical laboratories for the efficient and gentle removal of volatile solvents from samples. It operates by reducing the pressure within the system, which lowers the solvent's boiling point, allowing for rapid evaporation at a low temperature while rotation increases the liquid's surface area.
A rotovap doesn't just boil a solvent away; it manipulates the laws of physics to do so gently. By combining reduced pressure with rotation, it allows for rapid, efficient solvent removal at temperatures low enough to protect sensitive chemical compounds.

The Core Principle: Evaporation Under Reduced Pressure
To understand how a rotovap works, you must first grasp the relationship between pressure and boiling point. These two properties are directly linked.
Why Lower the Pressure?
Every liquid has a boiling point, the temperature at which its vapor pressure equals the pressure of the gas above it. At sea level (1 atm), water boils at 100°C.
However, if you decrease the surrounding pressure—as you would by climbing a mountain—the boiling point also decreases.
A rotovap exploits this principle by using a vacuum pump to create a low-pressure environment inside the apparatus. This dramatically lowers the solvent's boiling point, often to room temperature or slightly above.
The Role of the Vacuum System
The vacuum pump is the heart of the pressure reduction system. It actively removes air and solvent vapor from the glassware, creating and maintaining the low-pressure environment necessary for low-temperature evaporation.
A vacuum controller allows for precise regulation of this pressure, which is critical for targeting the boiling point of a specific solvent without causing the sample to foam or bump.
A Component-by-Component Breakdown of the Mechanism
Each part of the rotovap plays a distinct and crucial role in executing this process safely and efficiently.
The Heating Bath: Providing Gentle Energy
While the vacuum lowers the boiling point, evaporation still requires energy (the latent heat of vaporization). The heating bath, typically filled with water, provides this energy gently and uniformly.
The goal is not to aggressively boil the liquid but to supply just enough warmth to sustain evaporation at the new, lower boiling point. This low-temperature heating is what protects heat-sensitive compounds from degradation.
The Rotating Flask: Maximizing Surface Area and Preventing Bumping
The rotation of the sample flask is the rotovap's other key innovation. As the flask spins, it continuously spreads the sample into a thin film on the interior surface.
This has two major benefits. First, it vastly increases the surface area available for evaporation, making the process much faster. Second, the constant agitation and even heat distribution prevent "bumping," a phenomenon where superheated pockets of solvent erupt violently.
The Condenser: Recapturing the Solvent
Once the solvent evaporates, the resulting vapor travels into a condenser coil. This coil is kept cold by continuously circulating fluid, such as tap water or a dedicated chiller.
When the warm solvent vapor hits the cold glass surface of the condenser, it rapidly cools and liquefies, turning back into a liquid.
The Receiving Flask: Collecting the Distillate
Gravity then pulls the condensed liquid solvent down into a collection vessel known as the receiving flask. This allows for the clean separation and recovery of the solvent, which can often be reused.
Meanwhile, your non-volatile compound of interest is left behind as a solid or oil in the rotating flask, now free of solvent.
Understanding the Trade-offs and Common Pitfalls
While highly effective, operating a rotovap requires skill to avoid common problems that can compromise your sample or the process itself.
The Risk of Bumping and Foaming
If the pressure is reduced too quickly or the temperature is set too high for the chosen vacuum level, the sample can boil too vigorously. This can cause "bumping," where the sample violently erupts and contaminates the rest of the apparatus.
Some solutions can also foam, especially if they contain surfactants. This requires a very gradual application of vacuum to manage.
Protecting Your Sample
The primary goal is to remove the solvent without losing your product. If the vacuum is too strong (pressure is too low) or the bath is too hot, you risk co-distilling a semi-volatile compound along with the solvent.
You must choose parameters that are aggressive enough for the solvent but gentle enough for your compound of interest.
System Integrity and Safety
A rotovap operates glass components under vacuum, which carries an inherent risk of implosion if the glassware is damaged. Always inspect flasks for star cracks or chips before use.
Using the safety shield and wearing safety glasses are non-negotiable practices to protect against this potential hazard.
Making the Right Choice for Your Goal
Optimizing the process depends on balancing speed, sample safety, and solvent recovery based on your specific needs.
- If your primary focus is speed: Use a lower pressure and a slightly warmer bath temperature, but monitor the flask closely for signs of bumping.
- If your primary focus is protecting a fragile compound: Use a more moderate vacuum and the lowest possible bath temperature that still permits efficient evaporation.
- If your primary focus is maximizing solvent recovery: Ensure your condenser is sufficiently cold to efficiently recapture all vapor, preventing solvent loss into the vacuum pump and atmosphere.
Mastering these principles transforms the rotovap from a simple machine into a precise and powerful tool for chemical purification.
Summary Table:
| Component | Key Function |
|---|---|
| Vacuum Pump | Lowers pressure to reduce solvent boiling point |
| Heating Bath | Provides gentle heat for evaporation |
| Rotating Flask | Increases surface area and prevents bumping |
| Condenser | Cools vapor back into liquid for recovery |
| Receiving Flask | Collects the purified solvent |
Optimize Your Lab's Solvent Removal Process with KINTEK
Mastering rotary evaporation is key to efficient and safe chemical purification. KINTEK specializes in high-quality lab equipment, including reliable rotary evaporators designed for precise temperature and pressure control. Whether you're working with heat-sensitive compounds or need to maximize solvent recovery, our solutions enhance your lab's productivity and safety.
Let KINTEK provide the right equipment for your laboratory needs. Contact our experts today to discuss how our rotovaps and consumables can support your research and purification workflows.
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 30T 40T Split Automatic Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What types of gases can a water circulating vacuum pump handle? Safely Manage Flammable, Condensable & Dirty Gases
- How is a circulating water vacuum pump utilized for hydrogen production residues? Optimize Your Solid-Liquid Separation
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation
- What is the importance of a vacuum pump for Schottky hybrid interfaces? Achieve Atomic-Level Purity and Bonding
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits



















