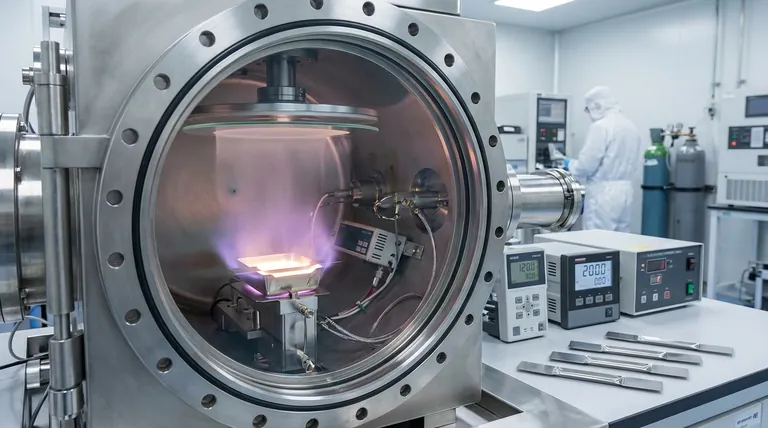
In essence, vacuum evaporation is a process that transforms a solid material into a thin, uniform film by heating it in a high-vacuum environment until it vaporizes. These vaporized particles then travel unimpeded and condense onto a cooler target surface, known as a substrate, forming the desired coating.
The core insight is that the vacuum is not just an incidental detail; it is the key enabler of the process. It lowers the boiling point of the source material and provides a clear, contaminant-free path for vapor particles to travel from the source to the substrate, ensuring a high-purity film.
The Core Principle: From Solid to Vapor to Film
Vacuum evaporation is one of the simplest forms of Physical Vapor Deposition (PVD), a family of processes where a material is converted into a vapor phase and then condensed to form a thin film.
The Role of the Vacuum
The process must take place in a high-vacuum chamber for two critical reasons. First, the vacuum drastically reduces the pressure, which in turn lowers the temperature at which the source material evaporates or sublimates.
Second, removing air molecules prevents the vaporized source particles from colliding with them. This ensures a direct, "line-of-sight" trajectory to the substrate, which is crucial for uniform deposition and preventing contamination of the final film.
The Evaporation and Condensation Cycle
The process is a physical transition from solid to vapor and back to solid. The source material is heated until its atoms gain enough energy to overcome their binding forces and enter a gaseous state.
These vapor particles travel through the vacuum and strike the cooler substrate. Upon impact, they lose their energy, condense, and nucleate, gradually building up layer by layer into a solid thin film.
How the Process Works Step-by-Step
A typical vacuum evaporation system consists of three main parts: the vacuum chamber, the evaporation source that heats the material, and the substrate holder.
The Energy Source
To create the vapor, the source material is heated. One common method is e-beam evaporation, where a focused beam of high-energy electrons is directed at the source material, which is held in a water-cooled crucible. The intense heat from the beam causes the material to vaporize.
The Line-of-Sight Trajectory
Once vaporized, the particles travel in straight lines away from the source. This characteristic allows for precise deposition on surfaces directly facing the source, almost like spray-painting with individual atoms.
Deposition on the Substrate
The substrate is strategically placed to intercept the flow of vaporized particles. As the particles land on the substrate, they form the desired thin film. The rate of deposition can be easily monitored and controlled by adjusting the heating power.
Understanding the Trade-offs
Like any technical process, vacuum evaporation has clear benefits and limitations that make it suitable for specific applications.
Key Advantages
This method is highly valued for its ability to produce high-purity films, as the high vacuum minimizes contaminants. It is also the least expensive PVD process, compatible with a wide range of source materials, and offers straightforward control over the deposition rate.
Inherent Limitations
The primary limitation is its line-of-sight nature. Because particles travel in straight lines, it is difficult to coat complex, three-dimensional shapes with undercuts or hidden surfaces. The film will be thickest on surfaces directly facing the source and absent on those that are shadowed.
Common Variations and Applications
The fundamental process can be adapted for different outcomes, ranging from simple metal coatings to complex wastewater purification.
Thin-Film Deposition
This is the most common application. It is used to create optical interference coatings, reflective mirror coatings, decorative films, and electrically conductive layers for electronics. When used with metals like aluminum, it is often called vacuum metallization.
Multi-Source Evaporation
For creating alloy or composite films, two or more evaporation sources can be used simultaneously. By precisely controlling the evaporation rate of each source, engineers can create films with a specific, mixed composition.
A Different Application: Wastewater Treatment
The same physical principle—evaporation under vacuum to lower the boiling point—is also used in wastewater treatment. This process efficiently separates clean water (the distillate) from contaminants with high boiling points (the concentrate).
Making the Right Choice for Your Goal
Selecting vacuum evaporation depends entirely on your specific material and application requirements.
- If your primary focus is high-purity, simple coatings on flat surfaces: Vacuum evaporation offers the most cost-effective and straightforward PVD solution.
- If your primary focus is creating precise alloy or composite films: A multi-source evaporation setup provides direct control over the final film composition.
- If your primary focus is coating complex 3D objects uniformly: You should consider a non-line-of-sight process like sputtering or chemical vapor deposition.
Ultimately, understanding the principles of vacuum evaporation empowers you to select a powerful and precise tool for material deposition and purification.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process Type | Physical Vapor Deposition (PVD) |
| Core Principle | Heating a material in a vacuum to vaporize it, then condensing it onto a substrate. |
| Key Advantage | High-purity films, cost-effective, simple rate control. |
| Primary Limitation | Line-of-sight nature; difficult for coating complex 3D shapes. |
| Common Applications | Optical coatings, reflective mirrors, conductive layers (metallization). |
Ready to achieve precise, high-purity thin films for your laboratory?
At KINTEK, we specialize in providing robust vacuum evaporation systems and consumables tailored to your R&D and production needs. Whether you're working on optical coatings, electronics, or specialized materials research, our expertise ensures you get the right equipment for reliable results.
Contact our experts today to discuss how our lab equipment can enhance your deposition processes and drive your projects forward.
Visual Guide
Related Products
- Molybdenum Tungsten Tantalum Evaporation Boat for High Temperature Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Aluminized Ceramic Evaporation Boat for Thin Film Deposition
People Also Ask
- Is brazing and soldering the same? Understand the Critical Temperature Difference for Stronger Joints
- What function does a silicon infiltration furnace perform in SiC/SiC bonding? Optimize Reactive Melt Infiltration
- What are the conditions for tempering? Master the Heat Treatment Process for Stronger Steel
- What is a low temperature form of brazing? Discover Solid-State Joining for Heat-Sensitive Materials
- What are the 4 heat treatments of steel? Master Hardness, Toughness & More
- What is the role of a laboratory high-temperature resistance furnace in TSR testing? Quantifying Material Durability
- At what temperature does annealing take place? A Guide to Material-Specific Heat Treatment
- Can you vacuum braze aluminum? A Guide to Flux-Free, High-Strength Joining



















