Determining the optimal temperature for a rotary evaporator, or Rotavap, is not about finding a single magic number. The correct temperature is entirely dependent on the solvent you are removing, the vacuum pressure you can achieve, and the thermal stability of your compound. The most common guideline is to set the heating bath temperature approximately 20°C higher than the boiling point of your solvent at the operating pressure.
The core principle is not to heat the sample to its atmospheric boiling point, but to lower the solvent's boiling point by applying a vacuum. The heat bath's role is simply to provide the energy needed for vaporization at this new, lower temperature.

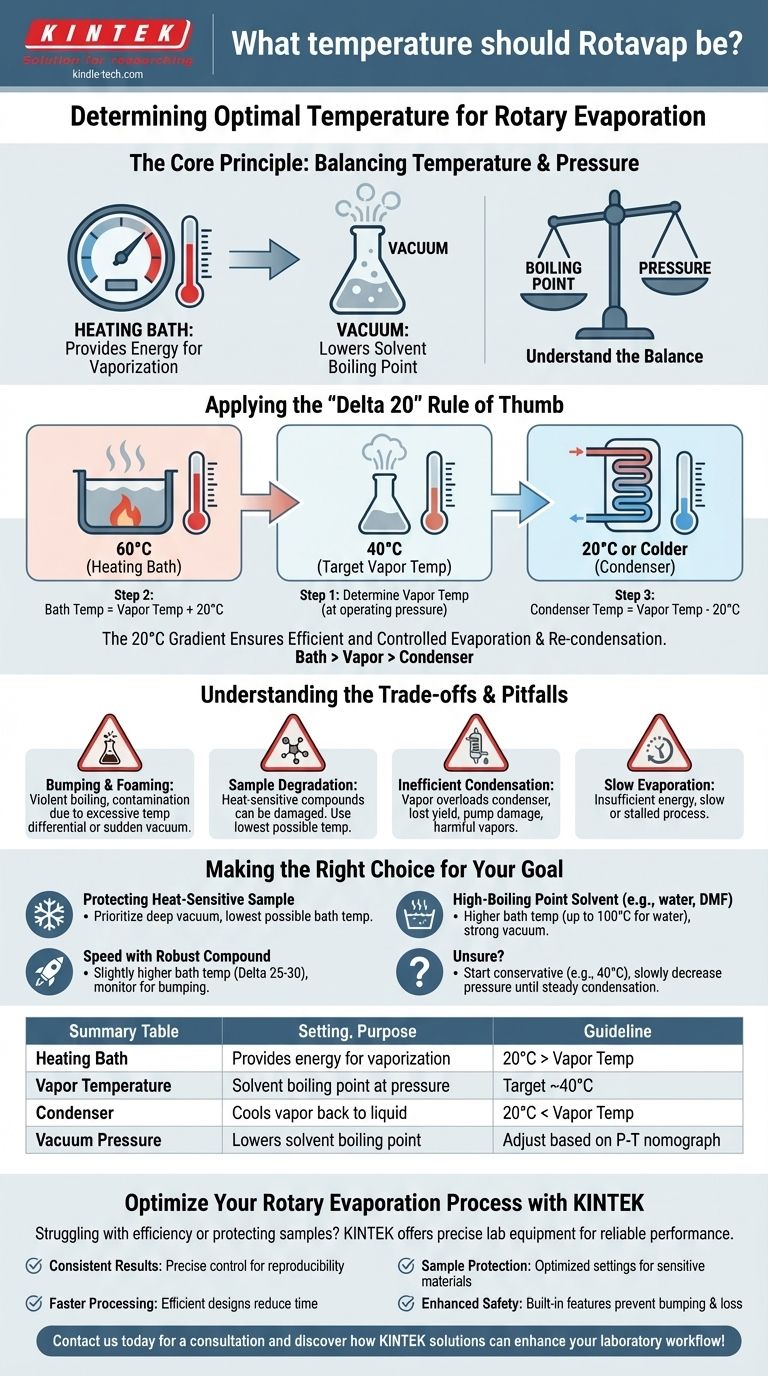
The Core Principle: Balancing Temperature and Pressure
A rotary evaporator works by manipulating the relationship between a liquid's boiling point and the pressure of the system. Understanding this balance is the key to effective use.
Why There's No Single "Right" Temperature
The boiling point of a liquid is the temperature at which its vapor pressure equals the pressure of the surrounding environment.
At sea level, water boils at 100°C. On a high mountain, where atmospheric pressure is lower, water boils at a lower temperature. A Rotavap exploits this same principle.
The Role of the Vacuum
Applying a vacuum with a pump drastically reduces the pressure inside the system.
This pressure reduction lowers the boiling point of your solvent, often significantly. This allows you to evaporate solvents like ethanol or ethyl acetate at room temperature or slightly above, protecting heat-sensitive compounds.
The Role of the Heating Bath
Once the vacuum has lowered the solvent's boiling point, the heating bath provides the thermal energy (known as the latent heat of vaporization) required for the phase change from liquid to gas.
Without the bath, the evaporation would draw energy from the solvent itself, causing it to cool down and eventually stop boiling. The bath ensures the process is continuous and efficient.
Applying the "Delta 20" Rule of Thumb
The "Delta 20 Rule" (or Δ20 Rule) is a widely accepted guideline for setting the three key temperatures in a rotary evaporation system.
The Three Temperatures
The rule states there should be a 20°C difference between each stage: the heating bath, the vapor, and the cooling condenser.
Bath > Vapor > Condenser
This temperature gradient ensures efficient and controlled evaporation and re-condensation.
Step 1: Determine Your Target Vapor Temperature
The vapor temperature is the boiling point of your solvent at the pressure you are using. You can find this using a pressure-temperature nomograph, which is a standard chart in most chemistry labs.
For many common organic solvents, a target vapor temperature of around 40°C provides a good balance of speed and safety.
Step 2: Set the Heating Bath Temperature
Following the Delta 20 rule, set your heating bath temperature 20°C higher than your target vapor temperature.
For a target vapor temperature of 40°C, you would set your heating bath to 60°C.
Step 3: Set the Condenser Temperature
Similarly, set your cooling liquid (circulating through the condenser) 20°C lower than your target vapor temperature.
For a target vapor temperature of 40°C, your coolant should be 20°C or colder. Standard tap water is often sufficient if it is cold enough.
Understanding the Trade-offs and Pitfalls
Setting the temperature is a balancing act. Deviating from the ideal setup can lead to inefficiency or dangerous situations.
Risk of Bumping and Foaming
If the temperature differential between the bath and the solvent's boiling point is too great, or if vacuum is applied too suddenly, the liquid can boil violently.
This is known as bumping. It can cause your solution to splash into the condenser and collection flask, contaminating your product and ruining the separation.
Risk of Sample Degradation
The primary reason to use a Rotavap is to handle heat-sensitive materials. Even a "low" bath temperature of 60°C can be too hot for highly unstable compounds. Always use the lowest possible temperature that allows for a reasonable evaporation rate.
Inefficient Condensation
If the heating bath is too hot, it can create vapor faster than the condenser can turn it back into a liquid. This overloads the condenser, causing solvent vapor to pass through into your vacuum pump.
This reduces yield, damages the pump, and releases potentially harmful solvent vapors into the laboratory.
Slow Evaporation
If the bath temperature is too low, you will not provide enough energy to maintain boiling. The evaporation will be exceedingly slow or may stall completely.
Making the Right Choice for Your Goal
Always prioritize the stability of your compound. Use the following guidelines to adjust your settings based on your primary objective.
- If your primary focus is protecting a heat-sensitive sample: Prioritize a deep vacuum to lower the boiling point as much as possible, and use the lowest corresponding bath temperature.
- If your primary focus is speed with a robust compound: Use a slightly higher bath temperature (e.g., Delta 25-30) for faster evaporation, but monitor the flask closely for any signs of bumping.
- If you are working with a high-boiling point solvent (like water or DMF): You will need a higher bath temperature (up to 100°C for water) and a strong vacuum to achieve an efficient rate of evaporation.
- If you are ever unsure: Start with a conservative bath temperature (e.g., 40°C) and slowly decrease the system pressure until you observe a steady rate of condensation on the condenser coils.
Mastering the interplay of temperature and pressure is the key to safe, efficient, and reproducible rotary evaporation.
Summary Table:
| Setting | Purpose | Guideline |
|---|---|---|
| Heating Bath | Provides energy for vaporization | 20°C higher than vapor temperature |
| Vapor Temperature | Solvent boiling point at operating pressure | Target ~40°C for common solvents |
| Condenser | Cools vapor back to liquid | 20°C lower than vapor temperature |
| Vacuum Pressure | Lowers solvent boiling point | Adjust based on solvent P-T nomograph |
Optimize Your Rotary Evaporation Process with KINTEK
Struggling with solvent removal efficiency or protecting heat-sensitive compounds? KINTEK specializes in precision lab equipment and consumables that deliver reliable performance for your laboratory needs. Our rotary evaporators feature precise temperature control and robust vacuum systems, ensuring safe and efficient solvent evaporation while protecting your valuable samples.
We help you achieve:
- Consistent Results: Precise temperature and pressure control for reproducible evaporation
- Sample Protection: Optimized settings for heat-sensitive materials
- Faster Processing: Efficient designs that reduce evaporation time
- Enhanced Safety: Built-in features to prevent bumping and solvent loss
Let our experts help you select the right equipment and optimize your evaporation parameters. Contact us today for a consultation and discover how KINTEK solutions can enhance your laboratory workflow!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Evaporation Crucible for Organic Matter
- Ceramic Evaporation Boat Set Alumina Crucible for Laboratory Use
- Hemispherical Bottom Tungsten Molybdenum Evaporation Boat
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
People Also Ask
- What are the drive types for variable speed peristaltic pumps? Electric vs. Pneumatic for Your Application
- Which is better blast furnace or electric arc furnace? Choose the Right Steelmaking Technology for Your Needs
- Does quartz have a high melting point? Discover Its Superior High-Temperature Performance
- What is the alternative to brazing? Compare Welding, Soldering & Mechanical Fastening
- What is sintering process in blast furnace? Transform Iron Ore Fines into High-Performance Feedstock
- Why is 100% sinter not used in blast furnace? Avoid Crippling Gas Flow & Instability
- How do you evaporate a high boiling point solvent? Master Low-Pressure Techniques to Protect Your Samples
- What is the temperature of thermal debinding? A Guide to Controlled Binder Removal Cycles



















