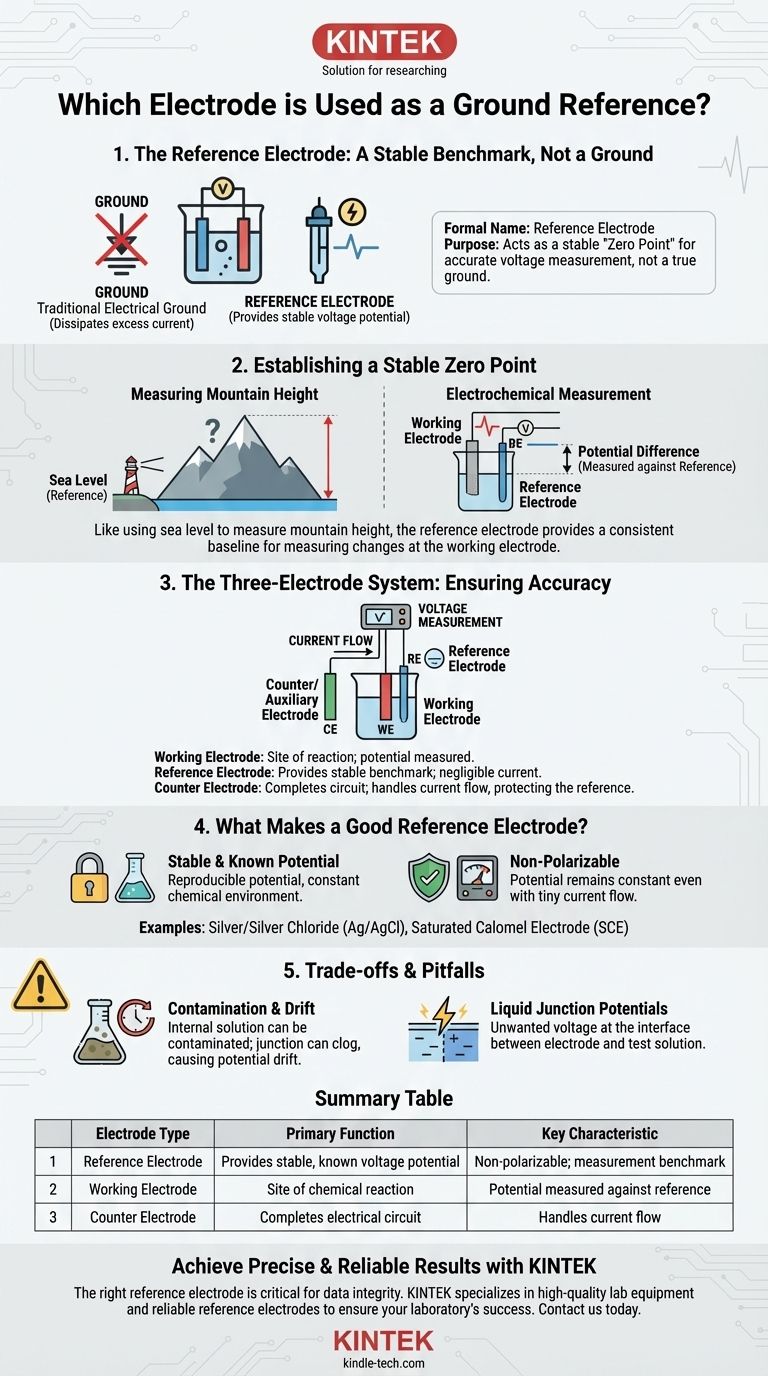
The electrode used as a ground reference is formally called a reference electrode. Its purpose is not to act as a true electrical ground, but to provide an exceptionally stable and well-known voltage potential. This stable voltage serves as the fixed "zero point" against which the unknown and often fluctuating potential of another electrode—the working electrode—can be accurately measured.
A reference electrode is not a ground in the traditional electrical sense. Instead, it is a specialized electrochemical half-cell that maintains a constant potential, acting as an essential and stable benchmark for all voltage measurements within the system.

The Role of the Reference Electrode
To understand why a reference electrode is critical, you must first understand that you cannot measure the absolute potential of a single electrode. You can only measure the potential difference between two of them.
Establishing a Stable Zero Point
Think of measuring the height of a mountain. You need a consistent baseline, like sea level, to get a meaningful number. The reference electrode acts as this "sea level."
It provides a constant voltage that does not change during your experiment. This allows you to accurately measure any changes happening at your primary electrode of interest, known as the working electrode.
The Three-Electrode System
Most modern electrochemical measurements use a three-electrode setup to ensure accuracy:
- Working Electrode: This is where the chemical reaction you are studying takes place. Its potential is what you want to measure.
- Reference Electrode: This is placed near the working electrode. Its sole job is to provide a stable reference potential for measurement. Almost no current flows through it.
- Counter (or Auxiliary) Electrode: This electrode completes the electrical circuit. It passes all the current needed for the reaction at the working electrode, ensuring the reference electrode remains undisturbed.
This separation of tasks is key. The counter electrode handles the disruptive flow of current, allowing the reference electrode to remain a pure, stable point of measurement.
What Makes a Good Reference Electrode?
Not just any piece of metal will work. A reference electrode must have specific properties to be effective.
A Stable and Known Potential
The most important characteristic is a potential that is stable, reproducible, and well-documented. This stability is achieved by using a carefully controlled redox system (a reversible chemical reaction) where the components are kept at a constant, saturated concentration.
Non-Polarizable
A reference electrode should be non-polarizable, meaning its potential does not change even if a tiny amount of current accidentally flows through it. This ensures it remains a steadfast benchmark throughout the measurement process.
Common Examples
While the Standard Hydrogen Electrode (SHE) is the theoretical standard with a potential defined as 0.00 V, it is impractical for most labs.
Instead, more common and robust reference electrodes are used, such as:
- Silver/Silver Chloride (Ag/AgCl): Highly common, stable, and relatively inexpensive.
- Saturated Calomel Electrode (SCE): Another very stable and historically popular choice, though it contains mercury.
Understanding the Trade-offs
Using the term "ground reference" can create confusion. It's essential to recognize the potential pitfalls and distinctions.
"Ground" vs. "Reference"
In a traditional circuit, a ground is a path for excess current to be safely dissipated, often connected to the earth.
A reference electrode's job is the opposite: to maintain a constant voltage by ensuring as little current as possible flows through it. Confusing these two roles can lead to incorrect experimental setups and flawed data.
Contamination and Drift
Reference electrodes are not perfect. The internal solution can become contaminated, or the junction that connects it to the main solution can become clogged. Over time, this can cause the potential to "drift," reducing measurement accuracy. They require proper storage and periodic validation.
Liquid Junction Potentials
When the solution inside the reference electrode differs from your test solution, a small but significant unwanted voltage can develop at the interface. This "liquid junction potential" is a source of error that must be considered in high-precision work.
Making the Right Choice for Your Goal
Your choice and implementation of a reference electrode depend entirely on your application's requirements for precision and stability.
- If your primary focus is academic research or analytics: You must use a standard, high-quality reference electrode like Ag/AgCl or SCE to ensure your results are reproducible and comparable to published data.
- If your primary focus is a low-cost sensor application: You may use a "pseudo-reference" electrode (like a simple silver wire), but you must accept that its potential may drift and will not be directly comparable to standard values.
- If your primary focus is bio-potential measurement (ECG/EEG): You will use a combination of a reference electrode for measurement and a separate "driven right leg" circuit that functions as an active ground to reduce electrical noise from the body.
By understanding the reference electrode's true role as a stable benchmark, you gain control over the accuracy and integrity of your electrochemical measurements.
Summary Table:
| Electrode Type | Primary Function | Key Characteristic |
|---|---|---|
| Reference Electrode | Provides a stable, known voltage potential | Non-polarizable; acts as a measurement benchmark |
| Working Electrode | Site of the chemical reaction being studied | Its potential is measured against the reference |
| Counter Electrode | Completes the electrical circuit | Handles current flow to protect the reference electrode |
Achieve precise and reliable results in your electrochemical experiments. The right reference electrode is critical for data integrity. KINTEK specializes in high-quality lab equipment and consumables, including reliable reference electrodes, to ensure your laboratory's success. Contact us today to find the perfect electrochemical solution for your specific application. Get in touch with our experts
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Copper Sulfate Reference Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What is the reference electrode for mercury mercurous sulfate? A Guide to Chloride-Free Electrochemistry
- Why and how should the electrodes of an electrolytic cell be calibrated? Ensure Reliable Results
- What are the general precautions for using a reference electrode? Ensure Stable Potentials for Accurate Data
- What are the characteristics of a saturated calomel electrode for neutral solutions? Understanding its stability and limitations.
- What is the reference electrode for mercury mercury chloride? Discover the Saturated Calomel Electrode (SCE)



















